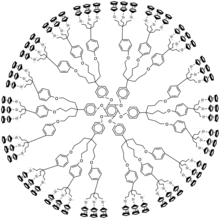
Metallodendrimer
[1][2][3] The metal can be situated in the repeat unit, the core or at the extremities as end-group.
These metals can form octahedral six-coordinate M(IV) linking units from organic dihalides and the corresponding 4-coordinate M(II) monomers.
Metallodendrimers can form as metal complexes with dendritic counter ions for example by hydrolysis of ester terminated PAMAM dendrimers with sodium hydroxide.
Applications can be expected in the fields of catalysis, as chemical sensors in molecular recognition - for example of bromine and chloride anions [4] - or as materials capable of binding metals.
Metallodendrimers can also mimic certain biomolecules for example haemoprotein in dendrimer with a porphyrin core.