Metallurgy
Metallurgy is a domain of materials science and engineering that studies the physical and chemical behavior of metallic elements, their inter-metallic compounds, and their mixtures, which are known as alloys.
[3] Modern metallurgists work in both emerging and traditional areas as part of an interdisciplinary team alongside material scientists and other engineers.
[4] In the late 19th century, metallurgy's definition was extended to the more general scientific study of metals, alloys, and related processes.
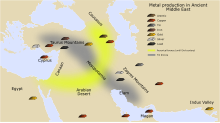
Small amounts of natural gold, dating to the late Paleolithic period, 40,000 BC, have been found in Spanish caves.
[5] Silver, copper, tin and meteoric iron can also be found in native form, allowing a limited amount of metalworking in early cultures.
[10][11][9] Certain metals, such as tin, lead, and copper can be recovered from their ores by simply heating the rocks in a fire or blast furnace in a process known as smelting.
The first evidence of copper smelting, dating from the 6th millennium BC,[12] has been found at archaeological sites in Majdanpek, Jarmovac and Pločnik, in present-day Serbia.
[13][8] The site of Pločnik has produced a smelted copper axe dating from 5,500 BC, belonging to the Vinča culture.
[14] The Balkans and adjacent Carpathian region were the location of major Chalcolithic cultures including Vinča, Varna, Karanovo, Gumelnița and Hamangia, which are often grouped together under the name of 'Old Europe'.
[17][18][19] The earliest documented use of lead (possibly native or smelted) in the Near East dates from the 6th millennium BC, is from the late Neolithic settlements of Yarim Tepe and Arpachiyah in Iraq.
[23][24] Other signs of early metals are found from the third millennium BC in Palmela, Portugal, Los Millares, Spain, and Stonehenge, United Kingdom.
[25][26] In the Near East, about 3,500 BC, it was discovered that by combining copper and tin, a superior metal could be made, an alloy called bronze.
Extractive metallurgists are interested in three primary streams: feed, concentrate (metal oxide/sulphide) and tailings (waste).
Concentrating the particles of value in a form supporting separation enables the desired metal to be removed from waste products.
Other engineering metals include aluminium, chromium, copper, magnesium, nickel, titanium, zinc, and silicon.
The task of the metallurgist is to achieve balance between material properties, such as cost, weight, strength, toughness, hardness, corrosion, fatigue resistance and performance in temperature extremes.
Metals can be heat-treated to alter the properties of strength, ductility, toughness, hardness and resistance to corrosion.
It involves bonding a thin layer of another metal such as gold, silver, chromium or zinc to the surface of the product.
[34] Thermal spraying techniques are another popular finishing option, and often have better high temperature properties than electroplated coatings.
These nonconductive surfaces include plastics, ceramics, and glass etc., which can then become decorative, anti-corrosive, and conductive depending on their final functions.
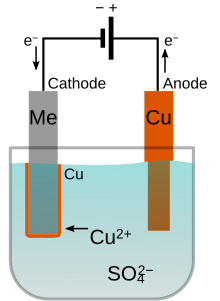
Metallurgists study the microscopic and macroscopic structure of metals using metallography, a technique invented by Henry Clifton Sorby.
The sample is then examined in an optical or electron microscope, and the image contrast provides details on the composition, mechanical properties, and processing history.