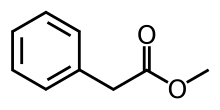
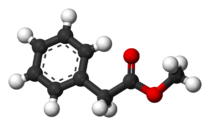
Methyl phenylacetate
Methyl phenylacetate is an organic compound that is the methyl ester of phenylacetic acid, with the structural formula C6H5CH2CO2CH3.
Methyl phenylacetate has a strong odor similar to honey.
This compound also occurs in brandy, capsicum, coffee, honey, pepper, and some wine.
It is used in the flavor industry and in perfumes to impart honey scents.
[1] Methyl phenyldiazoacetate, precursor to cyclopropanation agents, is prepared by treating methyl phenylacetate with p-acetamidobenzenesulfonyl azide in the presence of base.