Migratory insertion
In organometallic chemistry, a migratory insertion is a type of reaction wherein two ligands on a metal complex combine.
The insertion of carbon monoxide into a metal-carbon bond to form an acyl group is the basis of carbonylation reactions, which provides many commercially useful products.
Since square planar complexes are often coordinatively unsaturated, they are susceptible to formation of 5-coordinate adducts, which undergo migratory insertion readily.
[5] In most cases the in-plane migration pathway is preferred, but, unlike the nucleophilic pathway, it is inhibited by an excess of CO.[6] Decarbonylation of aldehydes, the reverse of CO insertion, is a well-recognized reaction: The reaction is not widely practiced in part because the alkanes are less useful materials than are the aldehyde precursors.
[9] Extrusion of CO from an organic aldehyde is most famously demonstrated using Wilkinson's catalyst:[10] Please see Tsuji-Wilkinson Decarbonylation Reaction for an example of this elementary organometallic step in synthesis Many electrophilic oxides insert into metal carbon bonds; these include sulfur dioxide, carbon dioxide, and nitric oxide.
The insertion of ethylene and propylene into titanium alkyls is the cornerstone of Ziegler–Natta catalysis, the main source of polyethylene and polypropylene.
The majority of this technology involves heterogeneous catalysts, but it is widely assumed that the principles and observations on homogeneous systems are applicable to the solid-state versions.
This transition state also highlights the two factors that most strongly contribute to the rate of olefin insertion reactions: (i) orbital overlap of the alkyl group initially attached to the metal and (ii) the strength of the metal-alkyl bond.
Analogous reactions apply to the hydrogenation of alkynes: an alkenyl ligand combines with a hydride to eliminate an alkene.
The Principle of Microscopic Reversibility requires that the mechanism of β-hydride elimination follow the same pathway as the insertion of alkenes into metal hydride bonds.
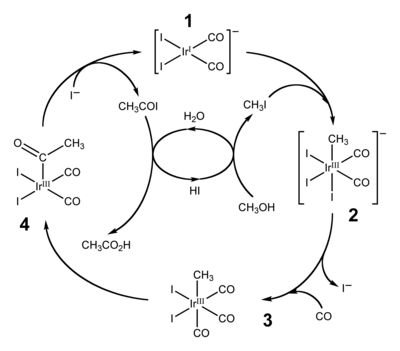
[15][16] By 2002, worldwide annual production of acetic acid stood at 6 million tons, of which approximately 60% is produced by the Cativa process.
The active catalyst species is regenerated by the reductive elimination of acetyl iodide from (4), a de-insertion reaction.
These steps can be repeated multiple times, potentially leading to high molecular weight polymers.