Molybdenum(II) acetate
It is a yellow, diamagnetic, air-stable solid that is slightly soluble in organic solvents.
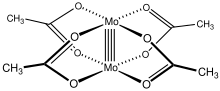
Molybdenum(II) acetate is an iconic example of a compound with a metal-metal quadruple bond.
[2] Like several other transition metal carboxylate complexes, Mo2(O2CCH3)4 adopts a Chinese lantern structure.
The Mo-O bond between each Mo(II) center and O atom from acetate has a distance of 2.119 Å, and the Mo-Mo distance between the two metal centers is 2.0934 Å. Mo2(O2CCH3)4 is prepared by treating molybdenum hexacarbonyl (Mo(CO)6) with acetic acid.
At the time, quadruple metal-metal bonds had not yet been discovered, so these authors proposed that Mo(O2CCH3)2 was tetrahedral.