Motor protein
Axonemal dynein, found in cilia and flagella, is crucial to cell motility, for example in spermatozoa, and fluid transport, for example in trachea.
The importance of motor proteins in cells becomes evident when they fail to fulfill their function.
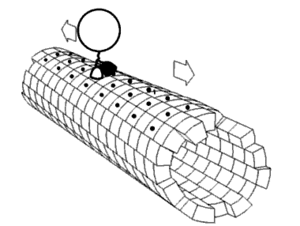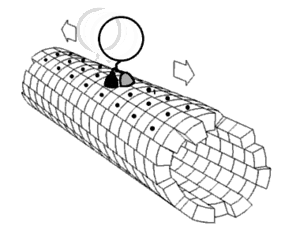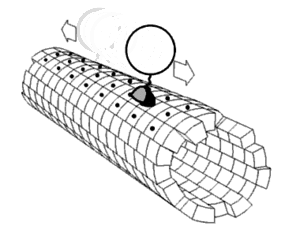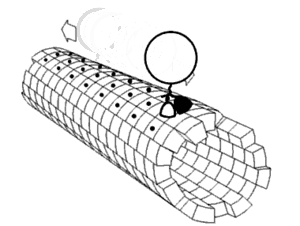
[1] Motor proteins utilizing the cytoskeleton for movement fall into two categories based on their substrate: microfilaments or microtubules.
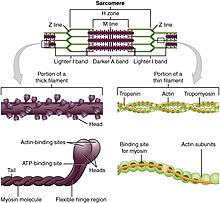
The myosin heads bind and hydrolyze ATP, which provides the energy to walk toward the plus end of an actin filament.
For example, myosin is involved in intracellular organization and the protrusion of actin-rich structures at the cell surface.
The two globular head motor domains in heavy chains can convert the chemical energy of ATP hydrolysis into mechanical work to move along microtubules.
In general, kinesins with N-terminal motor domains move their cargo towards the plus ends of microtubules located at the cell periphery, while kinesins with C-terminal motor domains move cargo towards the minus ends of microtubules located at the nucleus.
Axonemal dyneins facilitate the beating of cilia and flagella by rapid and efficient sliding movements of microtubules.
This process is facilitated by a phragmoplast, a microtubule array unique to plant cell mitosis.
The motor protein prestin,[14] expressed in mammalian cochlear outer hair cells, produces mechanical amplification in the cochlea.
It is a direct voltage-to-force converter, which operates at the microsecond rate and possesses piezoelectric properties.