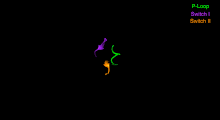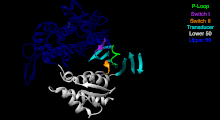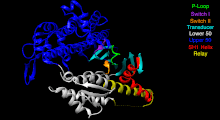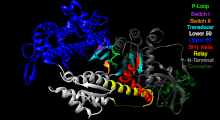Myosin
Following the discovery in 1973 of enzymes with myosin-like function in Acanthamoeba castellanii, a global range of divergent myosin genes have been discovered throughout the realm of eukaryotes.
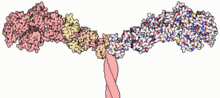
[5] Although myosin was originally thought to be restricted to muscle cells (hence myo-(s) + -in), there is no single "myosin"; rather it is a very large superfamily of genes whose protein products share the basic properties of actin binding, ATP hydrolysis (ATPase enzyme activity), and force transduction.
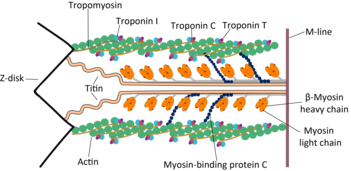
Multiple myosin II molecules generate force in skeletal muscle through a power stroke mechanism fuelled by the energy released from ATP hydrolysis.
The wide variety of myosin genes found throughout the eukaryotic phyla were named according to different schemes as they were discovered.
This protein makes up part of the sarcomere and forms macromolecular filaments composed of multiple myosin subunits.
Presumably this is so the myosins may interact, via their tails, with a large number of different cargoes, while the goal in each case – to move along actin filaments – remains the same and therefore requires the same machinery in the motor.
The hydrolysis of ATP and the subsequent release of the phosphate group causes the "power stroke", in which the "lever arm" or "neck" region of the heavy chain is dragged forward.
The velocity of a myosin motor depends upon the rate at which it passes through a complete kinetic cycle of ATP binding to the release of ADP.
[32]Myosin IV has a single IQ motif and a tail that lacks any coiled-coil forming sequence.
It has an extended lever arm consisting of five calmodulin binding IQ motifs followed by a single alpha helix (SAH)[46] Myosin VII is required for phagocytosis in Dictyostelium discoideum, spermatogenesis in C. elegans and stereocilia formation in mice and zebrafish.
[55] It is responsible for the light-directed movement of chloroplasts according to light intensity and the formation of stromules interconnecting different plastids.
[56] A specific Myosin XI found in Nicotiana tabacum was discovered to be the fastest known processive molecular motor, moving at 7μm/s in 35 nm steps along the actin filament.
[58] The myosins localize to plasma membranes of the intracellular parasites and may then be involved in the cell invasion process.
Known functions include: transporting phagosomes to the nucleus and perturbing the developmentally regulated elimination of the macronucleus during conjugation.
Myosin XV is necessary for the development of the actin core structure of the non-motile stereocilia located in the inner ear.
[62] Paramyosin is found in many different invertebrate species, for example, Brachiopoda, Sipunculidea, Nematoda, Annelida, Mollusca, Arachnida, and Insecta.
[61] Paramyosin is responsible for the "catch" mechanism that enables sustained contraction of muscles with very little energy expenditure, such that a clam can remain closed for extended periods.
A recent computational study showed that following human intestinal digestion, paramyosins of common octopus, Humboldt squid, Japanese abalone, Japanese scallop, Mediterranean mussel, Pacific oyster, sea cucumber, and Whiteleg shrimp could release short peptides that inhibit the enzymatic activities of angiotensin converting enzyme and dipeptidyl peptidase.