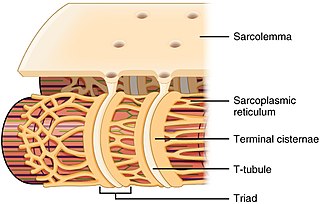
Sarcoplasmic reticulum
T-tubules are closely associated with a specific region of the SR, known as the terminal cisternae in skeletal muscle, with a distance of roughly 12 nanometers, separating them.
These calcium pumps are called Sarco(endo)plasmic reticulum Ca2+ ATPases (SERCA).
This shape change causes the cytosolic side of the pump to open, allowing the two Ca2+ to enter.
The cytosolic side of the pump then closes and the sarcoplasmic reticulum side opens, releasing the Ca2+ into the SR.[6] A protein found in cardiac muscle, called phospholamban (PLB) has been shown to prevent SERCA from working.
It does this by binding to the SERCA and decreasing its attraction (affinity) to calcium, therefore preventing calcium uptake into the SR. Failure to remove Ca2+ from the cytosol, prevents muscle relaxation and therefore means that there is a decrease in muscle contraction too.
When these hormones bind to a receptor, called a beta 1 adrenoceptor, located on the cell membrane, they produce a series of reactions (known as a cyclic AMP pathway) that produces an enzyme called protein kinase A (PKA).
PKA can add a phosphate to PLB (this is known as phosphorylation), preventing it from inhibiting SERCA and allowing for muscle relaxation.
This protein can bind to around 50 Ca2+, which decreases the amount of free Ca2+ within the SR (as more is bound to calsequestrin).
It is primarily located within the junctional SR/luminal space, in close association with the calcium release channel (described below).
Caffeine makes the RyR more sensitive to either the action potential (skeletal muscle) or calcium (cardiac or smooth muscle), thereby producing calcium sparks more often (this is partially responsible for caffeine's effect on heart rate).
The breakdown of the sarcoplasmic reticulum, along with the resultant release of calcium, is an important contributor to rigor mortis, the stiffening of muscles after death.