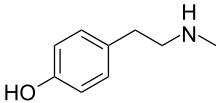
N-Methyltyramine
N-Methyltyramine (NMT), also known as 4-hydroxy-N-methylphenethylamine, is a human trace amine[1][2] and natural phenethylamine alkaloid found in a variety of plants.
[3] As the name implies, it is the N-methyl analog of tyramine, which is a well-known biogenic trace amine with which NMT shares many pharmacological properties.
One of the earliest syntheses is that reported by Walpole, who made it by the following sequence of steps: (i) acetylation of 4-methoxyphenethylamine with acetic anhydride; (ii) methylation of the amide using Na/methyl iodide; (iii) cleavage of the methyl ether to the phenol using HI; (iv) hydrolysis of the N-acetyl group with aqueous HCl.
Walpole also described an alternative, but similar sequence of reactions leading to NMT, beginning with the conversion of 4-methoxyphenethylamine to its benzenesulfonamide, which was then N-methylated and de-protected.
[15] A different method for making NMT was given by Corti, who prepared it by the thermal decarboxylation of N-methyltyrosine (ratanhin), by heating the amino-acid in fluorene at 250 °C.
[20] Subcutaneous administration of 10 mg/kg of the HCl salt of NMT to mice enhanced the release of norepinephrine (NE) from the heart by 36% over control, measured after 2 hours.
[21] A qualitatively similar decrease in the NE content of rat heart after treatment with NMT was observed by Camp.
[22] These researchers used a bio-assay-guided isolation procedure to show that NMT was the constituent of beer that was responsible for producing enhanced gastrin release, which in turn raises gastric acid secretion.
[28] The pharmacokinetics of NMT have been studied in rabbits and mice using drug that had been radiolabeled with tritium at C-3 and C-5 on the benzene ring.
Within 2 minutes of injection, significant levels of radioactivity were detected in all tissues examined, with the highest amounts being in kidney and liver.
Some NMT was found in the brains of mice treated with the drug, indicating that a small amount did cross the blood–brain barrier.
[18] Another acute toxicity study of NMT (under the Sterling-Winthrop company code "WIN 5582") found it to have an LD50 = 275 mg/kg, after intravenous administration to mice.