N95 respirator
An example of an early respirator standard, Type A, established in 1926, was intended to protect against mechanically generated dusts produced in mines.
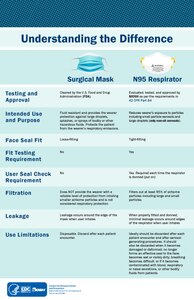
[30] Fit testing is a critical component to a respiratory protection program whenever workers use tight-fitting respirators in a hazardous environment.
[32] In the United States medical evaluation is required once, prior to initial fit testing and use, although it may need to be repeated if any adverse signs or symptoms are observed.
[42] In industrial settings where infectious disease exposure is not a concern, users can wear and reuse a filtering facepiece respirator until it is damaged, soiled, or causing noticeably increased breathing resistance, unless there is a manufacturer-specified duration of use.
[44] The same study found that "[m]odifications [such as the use of an electrocardiogram pad or surgical tape secured over the valve from the inside of the FFR] [...] can further reduce particle emissions".
[37] In the United States, the Occupational Safety and Health Administration (OSHA) requires healthcare workers who are expected to perform patient activities with those suspected or confirmed to be infected with COVID-19 to wear respiratory protection, such as an N95 respirator.
[31] During crisis situations where there is a shortage of N95 respirators, such as the COVID-19 pandemic, the U.S. Centers for Disease Control and Prevention (CDC) has recommended strategies for optimizing their use in healthcare settings.
[49] N95 respirators can be used beyond their manufacturer-designated shelf life, although components such as the straps and nose bridge material may degrade, making it particularly important that the wearer perform the expected seal check.
Portable fans with HEPA filters may also be used to increase ventilation in isolation rooms when surgical masks are being used in place of respirators.
[55][56][57] Duke University researchers have published a method for cleaning N95 respirators without damaging them using vaporized hydrogen peroxide to allow reuse for a limited number of times.
[53] A surgical mask is a loosely-placed, unsealed barrier, meant to stop droplets, and other liquid-borne particles from the mouth and nose that may contain pathogens.
[63] A CDC study found that in public indoor settings, consistently wearing a respirator was linked to a 83% lower risk of testing positive for COVID-19, as compared to a 66% reduction when using surgical masks, and 56% for cloth.
[64] While NIOSH was busy finishing 42 CFR 84 respirator regulations (including the N95), other agencies and groups (such as the SEIU[65]) were advocating for new standards for the prevention of TB.
The group was especially concerned about the rise of multidrug-resistant tuberculosis, which would require more rigorous standards to mitigate, especially since they felt that the 1990 CDC guidelines for TB were not being properly followed.
The proposed rule would require signage that includes a STOP sign, with red background, white symbols, and a set of words warning people to wear "N95 or more protective" respirators (under 42 CFR 84) near isolation rooms where TB infection is likely.
[C7] NIOSH certifies B Readers, people qualified to testify or provide evidence in mesothelioma personal injury lawsuits,[67] in addition to regulating respirators.
Combined with testimony that the plaintiff rarely wore a respirator around asbestos, the lack of evidence, and the limitation of liability from static NIOSH approval, the case was overturned.
[69] Despite this advice, a patient who had traveled from Ontario exposed six healthcare workers in Pennsylvania following contact tracing by the CDC, though fitted N95 respirators were worn at a hospital upon suspicion of SARS.
[71] Meanwhile, in Canada, discussions with Ontario EMS and New York Department of Health in 2004 noted that infected emergency medical personnel failed to properly use N95 respirators.
However, the report concludes, from laws preceding SARS, healthcare workers were obligated to wear N95 respirators throughout the outbreak, despite suggestions to the contrary.
[S2] A paper published in the New England Journal of Medicine concluded that universal use of N95 respirators, as well as additional infection control measures, ended the SARS outbreak in Ontario.
[73] In 2007, the CDC HICPAC published a set of guidelines, called the 2007 Guideline for Isolation Precautions: Preventing Transmission of Infectious Agents in Healthcare Settings, suggesting that use of "barrier precautions", defined as "masks, gowns, [and] gloves", would not be required, so long as it was limited to "routine entry", patients were not confirmed to be infected, and no aerosol-generating procedures were being done.
"Standard precautions" requiring the use of masks, face shields, and/or eye protection, would be needed if there was potential for the spraying of bodily fluids, like during intubation.
[89] Respirators came to be in short supply and high demand during the COVID-19 pandemic, causing price gouging and hoarding, often leading to confiscation of masks.
[90][91][92] Production of N95 respirators was limited due to constraints on the supply of nonwoven polypropylene fabric as well as the cessation of exports from China.
[1][93] Also in early April 2020, the United States federal government, invoking the DPA, ordered 3M to stop exporting N95 respirators to customers in Canada and Latin America, and to keep them within the U.S. instead.
Use of masks for source control is still recommended in times of high viral activity, but the CDC did not provide numbers for benchmarks.
The new policies are thought, according to the New York Times, based on various citations to medical literature, to increase mortality among vulnerable patients, especially those with cancer.
The 2023 paper also cites a research letter published in 2022, that suggests that the surge of COVID-19 cases in hospitals may have been due to the high contagiousness of Omicron,[97] an article which suggested a high secondary attack rate relative to Delta,[98] and papers finding increased mortality of cancer patients due to higher rates of breakthrough infections.
[102] In addition, in 2024, a paper published in the American Society for Microbiology Clinical Microbiology Reviews stated there were harms in continued undue weight being placed on RCTs and flawed mask studies in a social and political context, as retracted papers continue to be circulated to justify certain masking behaviors and beliefs.