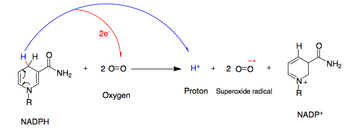
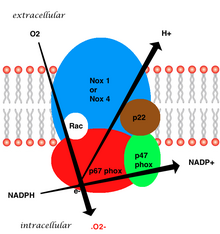
NADPH oxidase
The NADPH oxidase complex is dormant under normal circumstances but is activated to assemble in the membranes during respiratory burst.
The activated NADPH oxidase generates superoxide which has roles in animal immune response and plant signalling.
[13] It may also inactivate critical metabolic enzymes, initiate lipid peroxidation, damage iron-sulphur clusters,[14] and liberate redox-active iron, which allows the generation of indiscriminate oxidants such as the hydroxyl radical.
[16][17][18] In the gut, DUOX-dependent ROS production from bacteria-stimulated Drosophila melanogaster mucosa is an important pathogen-killing mechanism[19] and can increase defecation as a defense response.
[23][24] Careful regulation of NADPH oxidase activity is crucial to maintain a healthy level of ROS in the body.
Excessive production of ROS in vascular cells causes many forms of cardiovascular disease including hypertension, atherosclerosis, myocardial infarction, and ischemic stroke.
ROS produced by NADPH oxidase activate an enzyme that makes the macrophages adhere to the artery wall (by polymerizing actin fibers).
In vitro studies have found that the NADPH oxidase inhibitors apocynin and diphenyleneiodonium, along with the antioxidants N-acetyl-cysteine and resveratrol, depolymerized the actin, broke the adhesions, and allowed foam cells to migrate out of the intima.
These effects are in part responsible for inducing pre-eclampsia in pregnant women[34] Mutations in the NADPH oxidase subunit genes cause several Chronic Granulomatous Diseases (CGD), characterized by extreme susceptibility to infection.
NO donor drugs (nitrovasodilators) have therefore been used for more than a century to treat coronary artery disease, hypertension, and heart failure by preventing excess superoxide from deteriorating healthy vascular cells.
[36] The compound was initially developed for Idiopathic pulmonary fibrosis and obtained orphan drug designation by the FDA and EMA at end of 2010.