NIH shift
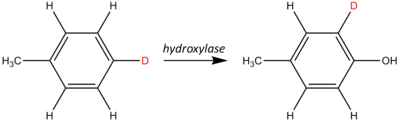
An NIH shift is a chemical rearrangement where a hydrogen atom on an aromatic ring undergoes an intramolecular migration primarily during a hydroxylation reaction.
These shifts are often studied and observed by isotopic labeling.
As the hydroxylase adds a hydroxyl (the −OH group), the labeled site shifts one position around the aromatic ring relative to the stationary methyl group (−CH3).
Several hydroxylase enzymes are believed to incorporate an NIH shift in their mechanism, including 4-hydroxyphenylpyruvate dioxygenase and the tetrahydrobiopterin dependent hydroxylases.
The name NIH shift arises from the US National Institutes of Health from where studies first reported observing this transformation.