Nafion
Nafion is a brand name for a sulfonated tetrafluoroethylene based fluoropolymer-copolymer synthesized in 1962 by Dr. Donald J. Connolly at the DuPont Experimental Station in Wilmington Delaware (U.S. Patent 3,282,875).
The dissociated protons "hop" from one acid site to another through mechanisms facilitated by the water molecules and hydrogen bonding.
Nafion can be manufactured with or exchanged to alternate cation forms for different applications (e.g. lithiated for Li-ion batteries) and at different equivalent weights (EWs), alternatively considered as ion-exchange capacities (IECs), to achieve a range of cationic conductivities with trade-offs to other physicochemical properties such as water uptake and swelling.
Nafion-H, for example, includes the following systematic names: The molecular weight of Nafion is variable due to differences in processing and solution morphology.
[3][4] The structure of a Nafion unit illustrates the variability of the material; for example, the most basic monomer contains chain variation between the ether groups (the z subscript).
Nafion can be dispersed into solution by heating in aqueous alcohol at 250 °C in an autoclave for subsequent casting into thin films or use as polymeric binder in electrodes.
[4] A cylindrical-water channel model[13] was also proposed based on simulations of small-angle X-ray scattering data and solid state nuclear magnetic resonance studies.
In this model, the sulfonic acid functional groups self-organize into arrays of hydrophilic water channels, each ~ 2.5 nm in diameter, through which small ions can be easily transported.
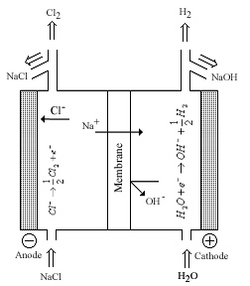
Nafion has found use in fuel cells, electrochemical devices, chlor-alkali production, metal-ion recovery, water electrolysis, plating, surface treatment of metals, batteries, sensors, Donnan dialysis cells, drug release, gas drying or humidification, and superacid catalysis for the production of fine chemicals.
The disadvantages of these systems is worker safety and environmental concerns associated with mercury and asbestos, economical factors also played a part, and in the diaphragm process chloride contamination of the hydroxide product.
[3] Although fuel cells have been used since the 1960s as power supplies for satellites, recently they have received renewed attention for their potential to efficiently produce clean energy from hydrogen.
Solid Polymer Electrolytes, which are made by connecting or depositing electrodes (usually noble metal) to both sides of the membrane, conduct the electrons through an energy requiring process and rejoin the hydrogen ions to react with oxygen and produce water.
Studies have demonstrated catalytic properties in alkylation, isomerization, oligomerization, acylation, ketalization, esterification, hydrolysis of sugars and ethers, and oxidation.
Nafion has been shown to be stable in cell cultures as well as the human body, and there is considerable research towards the production of higher sensitivity glucose sensors.
This limitation troubles the design of fuel cells because higher temperatures are desirable for better efficiency and CO tolerance of the platinum catalyst.
Silica and zirconium phosphate can be incorporated into Nafion water channels through in situ chemical reactions to increase the working temperature to above 100 °C.