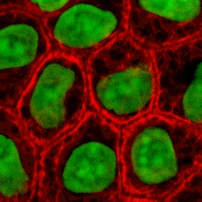
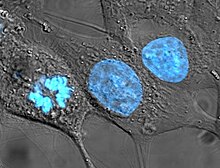
Cell culture
[1] These conditions vary for each cell type, but generally consist of a suitable vessel with a substrate or rich medium that supplies the essential nutrients (amino acids, carbohydrates, vitamins, minerals), growth factors, hormones, and gases (CO2, O2), and regulates the physio-chemical environment (pH buffer, osmotic pressure, temperature).
[3][4] The 19th-century English physiologist Sydney Ringer developed salt solutions containing the chlorides of sodium, potassium, calcium and magnesium suitable for maintaining the beating of an isolated animal heart outside the body.
[5] In 1885 Wilhelm Roux removed a section of the medullary plate of an embryonic chicken and maintained it in a warm saline solution for several days, establishing the basic principle of tissue culture.
[7][8][9] Ross Granville Harrison, working at Johns Hopkins Medical School and then at Yale University, published results of his experiments from 1907 to 1910, establishing the methodology of tissue culture.
Since Haberlandt's original assertions, methods for tissue and cell culture have been realized, leading to significant discoveries in biology and medicine.
A 1988 NIH SBIR grant report showed that electrospinning could be used to produce nano- and submicron-scale polymeric fibrous scaffolds specifically intended for use as in vitro cell and tissue substrates.
An established or immortalized cell line has acquired the ability to proliferate indefinitely either through random mutation or deliberate modification, such as artificial expression of the telomerase gene.
For the majority of isolated primary cells, they undergo the process of senescence and stop dividing after a certain number of population doublings while generally retaining their viability (described as the Hayflick limit).
In addition, chemically defined media can be used to eliminate any serum trace (human or animal), but this cannot always be accomplished with different cell types.
There are also cell lines that have been modified to be able to survive in suspension cultures so they can be grown to a higher density than adherent conditions would allow.
Adherent cells require a surface, such as tissue culture plastic or microcarrier, which may be coated with extracellular matrix (such as collagen and laminin) components to increase adhesion properties and provide other signals needed for growth and differentiation.
[38] A systematic bias in generated datasets was recently shown for CRISPR and RNAi gene silencing screens,[39] and for metabolic profiling of cancer cell lines.
Cell lines that originate with humans have been somewhat controversial in bioethics, as they may outlive their parent organism and later be used in the discovery of lucrative medical treatments.
Non-immortalized cells stop dividing after 40 to 60 population doublings[43] and, after this, they lose their ability to proliferate (a genetically determined event known as senescence).
[46] Biological products produced by recombinant DNA (rDNA) technology in animal cell cultures include enzymes, synthetic hormones, immunobiologicals (monoclonal antibodies, interleukins, lymphokines), and anticancer agents.
Mammalian cells ensure expressed proteins are folded correctly and possess human-like glycosylation and post-translational modifications.
Julius Richard Petri, a German bacteriologist, is generally credited with this invention while working as an assistant to Robert Koch.
Aside from Petri dishes, scientists have long been growing cells within biologically derived matrices such as collagen or fibrin, and more recently, on synthetic hydrogels such as polyacrylamide or PEG.
[69] There are a variety of platforms used to facilitate the growth of three-dimensional cellular structures including scaffold systems such as hydrogel matrices[70] and solid scaffolds, and scaffold-free systems such as low-adhesion plates, nanoparticle facilitated magnetic levitation,[71] hanging drop plates,[72][73] and rotary cell culture.
Culturing cells in 3D leads to wide variation in gene expression signatures and partly mimics tissues in the physiological states.
[76] Eric Simon, in a 1988 NIH SBIR grant report, showed that electrospinning could be used to produce nano- and submicron-scale polystyrene and polycarbonate fibrous scaffolds specifically intended for use as in vitro cell substrates.
This early use of electrospun fibrous lattices for cell culture and tissue engineering showed that various cell types including Human Foreskin Fibroblasts (HFF), transformed Human Carcinoma (HEp-2), and Mink Lung Epithelium (MLE) would adhere to and proliferate upon polycarbonate fibers.
[77] Hydrogels are composed of interconnected pores with high water retention, which enables efficient transport of substances such as nutrients and gases.
Tissue engineering potentially offers dramatic improvements in low cost medical care for hundreds of thousands of patients annually.
Due to the H5N1 pandemic threat, research into using cell culture for influenza vaccines is being funded by the United States government.
Novel ideas in the field include recombinant DNA-based vaccines, such as one made using human adenovirus (a common cold virus) as a vector,[78][79] and novel adjuvants.
The co-culture system in 3D models can predict the response to chemotherapy and endocrine therapy if the microenvironment defines biological tissue for the cells.
Microfluidics chip are also known as Lab-on-a-chip and they are able to have continuous procedure and reaction steps with spare amount of reactants and space.
Such systems enable the identification and isolation of individual cells and molecules when combined with appropriate biological assays and high-sensitivity detection techniques.
Combining tissue engineering, biomaterials fabrication, and cell biology, it offers the possibility of establishing a biomimetic model for studying human diseases in the laboratory.