Neon
[14] Neon is a colorless, odorless, inert monatomic gas under standard conditions, with approximately two-thirds the density of air.
Neon was discovered in 1898 alongside krypton and xenon, identified as one of the three remaining rare inert elements in dry air after the removal of nitrogen, oxygen, argon, and carbon dioxide.
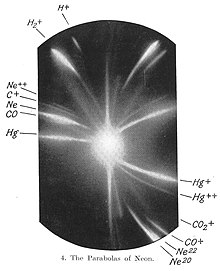
Its discovery was marked by the distinctive bright red emission spectrum it exhibited, leading to its immediate recognition as a new element.
The name neon originates from the Greek word νέον, a neuter singular form of νέος (neos), meaning 'new'.
The high volatility of neon and its inability to form compounds that would anchor it to solids explain its limited presence on Earth and the inner terrestrial planets.
Neon’s high volatility facilitated its escape from planetesimals under the early Solar System's nascent Sun's warmth.
This gas, identified in June, was named "neon", the Greek analogue of the Latin novum ('new')[17] suggested by Ramsay's son.
"[18] A second gas was also reported along with neon, having approximately the same density as argon but with a different spectrum – Ramsay and Travers named it metargon.
[19] Neon's scarcity precluded its prompt application for lighting along the lines of Moore tubes, which used nitrogen and which were commercialized in the early 1900s.
After 1902, Georges Claude's company Air Liquide produced industrial quantities of neon as a byproduct of his air-liquefaction business.
Claude tried briefly to sell neon tubes for indoor domestic lighting, due to their intensity, but the market failed because homeowners objected to the color.
In 1912, Claude's associate began selling neon discharge tubes as eye-catching advertising signs and was instantly more successful.
[20] The intense color and vibrancy of neon equated with American society at the time, suggesting a "century of progress" and transforming cities into sensational new environments filled with radiating advertisements and "electro-graphic architecture".
[23][24] The principal nuclear reactions generating nucleogenic neon isotopes start from 24Mg and 25Mg, which produce 21Ne and 22Ne respectively, after neutron capture and immediate emission of an alpha particle.
[24] Neon content observed in samples of volcanic gases and diamonds is also enriched in 20Ne, suggesting a primordial, possibly solar origin.
[33] Its relative rarity on Earth, like that of helium, is due to its relative lightness, high vapor pressure at very low temperatures, and chemical inertness, all properties which tend to keep it from being trapped in the condensing gas and dust clouds that formed the smaller and warmer solid planets like Earth.
[3] On 17 August 2015, based on studies with the Lunar Atmosphere and Dust Environment Explorer (LADEE) spacecraft, NASA scientists reported the detection of neon in the exosphere of the moon.
It is inert: as is the case with its lighter analog, helium, no strongly bound neutral molecules containing neon have been identified.
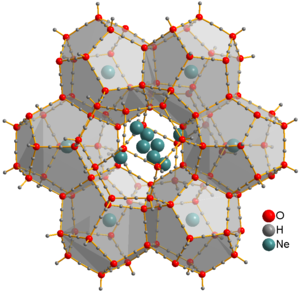
They can be extracted by placing the clathrate into a vacuum chamber for several days, yielding ice XVI, the least dense crystalline form of water.
[38] The familiar Pauling electronegativity scale relies upon chemical bond energies, but such values have obviously not been measured for inert helium and neon.
[46] As of 2020[update], the company Iceblick, with plants in Odesa and Moscow, supplies 65% of the world's production of neon, as well as 15% of the krypton and xenon.
[52][53] Neon signs typically operate at much higher voltages (2–15 kilovolts), and the luminous tubes are commonly meters long.