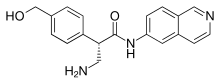
Netarsudil
[1][2][3] In the United States, in December 2017, the Food and Drug Administration (FDA) approved a 0.02% ophthalmic solution for the lowering of elevated intraocular pressure in people with open-angle glaucoma or ocular hypertension.
[2][8] Overdosing netarsudil is unlikely because concentrations in the body are so low that they are generally not detectable.
This appears to increase outflow of aqueous humor through the trabecular meshwork, and also to reduce pressure in the veins of the episcleral layer.
[2][7] After instillation into the eye, netarsudil is cleaved by esterases in the cornea to AR-13503, which is the active metabolite.
[7] The drug is used in form of a salt, netarsudil dimesilate, which is a white to light yellow crystalline powder.