Neuroinflammation
It may be initiated in response to a variety of cues, including infection, traumatic brain injury,[1] toxic metabolites, or autoimmunity.
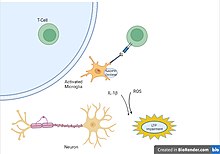
[2] In the central nervous system (CNS), including the brain and spinal cord, microglia are the resident innate immune cells that are activated in response to these cues.
[4] Although the response is initiated to protect the central nervous system from the infectious agent, the effect may be toxic and widespread inflammation as well as further migration of leukocytes through the blood–brain barrier may occur.
[5] Acute inflammation usually follows injury to the central nervous system immediately, and is characterized by inflammatory molecules, endothelial cell activation, platelet deposition, and tissue edema.
Some of these foreign pathogens can trigger a strong inflammatory response that can compromise the integrity of the blood-brain barrier and thus change the flow of inflammation in nearby tissue.
During this time, microglia generate reactive oxygen species and release signals to recruit peripheral immune cells for an inflammatory response.
In the healthy brain, cells secrete cytokines to produce a local inflammatory environment to recruit microglia and clear the infection or injury.
[6] After injury and sustained release of inflammatory factors such as chemokines, the blood–brain barrier may be compromised, becoming permeable to circulating blood components and peripheral immune cells.
This can result in sustained brain damage as anti-inflammatory factors decrease in amount when more pro-inflammatory cytokines are produced in excess by microglia.
[15] A primary SCI is caused by spinal cord compression or transection, leading to glutamate excitotoxicity, sodium and calcium ion imbalances, and free radical damage.
[15] This leads to a secondary SCI, whose symptoms include edema, cavitation of spinal parenchyma, reactive gliosis, and potentially permanent loss of function.
[15] During the SCI-induced inflammatory response, several pro-inflammatory cytokines including interleukin 1β (IL-1β), inducible nitric oxide synthase (iNOS), interferon-γ (IFN-γ), IL-6, IL-23, and tumor necrosis factor α (TNFα) are secreted, activating local microglia and attracting various immune cells such as naive bone-marrow derived macrophages.
[24] As one of the major cytokines responsible for maintaining inflammatory balance, IL-6 can also be used as a biological marker to observe the correlation between age and neuroinflammation.
Current thinking in AD pathology goes beyond these two typical hallmarks to suggest that a significant portion of neurodegeneration in Alzheimer's is due to neuroinflammation.
Current thought is that inflammatory cytokine-activated microglia cannot phagocytose amyloid-beta, which may contribute to plaque accumulation as opposed to clearance.
The inflammatory response in the gut may play a role[citation needed] in alpha-synuclein (α-Syn) aggregation and misfolding, a characteristic of Parkinson's disease pathology.
In Stage 3 of the hypothesis, the inflammation affects the substantia nigra, the dopamine producing cells of the brain, beginning the characteristic motor deficits of Parkinson's disease.
Stage 4 of Parkinson's disease includes deficits caused by inflammation in key regions of the brain that regulate executive function and memory.
Replacement of mSOD1 microglia and astrocytes with the wild-type forms delayed motor neuron (MN) degeneration and extended the lifespan of ALS mice.
Activation[44] and invasion[45][46] of peripheral monocytes observed in the spinal cord of ALS patients and mice may lead to MN loss.
Expression of several complement components are reported to be upregulated in the samples isolated from ALS patients[47] and transgenic rodent models.
[50] In multiple sclerosis, inflammatory cytokines disrupt the blood–brain barrier and allow for the migration of peripheral immune cells into the central nervous system.
[30] Because neuroinflammation has been associated with a variety of neurodegenerative diseases, there is increasing interest to determine whether reducing inflammation will reverse neurodegeneration.
[23] Aerobic exercise is used widely to reduce inflammation in the periphery by activating protective systems in the body that stabilize internal environment.
The ability of physical activity to stimulate immune defenses against neuroinflammation-related diseases has been observed in recent clinical studies.