Neurotoxin
[15] Macroscopic manifestations of neurotoxin exposure can include widespread central nervous system damage such as intellectual disability,[5] persistent memory impairments,[16] epilepsy, and dementia.
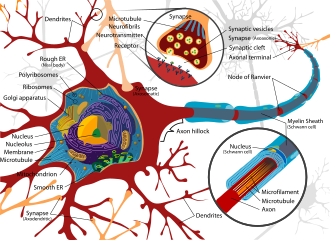
The nervous tissue found in the brain, spinal cord, and periphery comprises an extraordinarily complex biological system that largely defines many of the unique traits of individuals.
The choroid plexuses are vascularized layers of tissue found in the third, fourth, and lateral ventricles of the brain, which through the function of their ependymal cells, are responsible for the synthesis of cerebrospinal fluid (CSF).
[23] Importantly, through selective passage of ions and nutrients and trapping heavy metals such as lead, the choroid plexuses maintain a strictly regulated environment which contains the brain and spinal cord.
In modern times, scientists and physicians have been presented with the challenge of identifying and treating neurotoxins, which has resulted in a growing interest in both neurotoxicology research and clinical studies.
[24] Though clinical neurotoxicology is largely a burgeoning field, extensive inroads have been made in the identification of many environmental neurotoxins leading to the classification of 750 to 1000 known potentially neurotoxic compounds.
[21] Due to the critical importance of finding neurotoxins in common environments, specific protocols have been developed by the United States Environmental Protection Agency (EPA) for testing and determining neurotoxic effects of compounds (USEPA 1998).
[26] In an effort to address this complication, neurite outgrowths (either axonal or dendritic) in response to applied compounds have recently been proposed as a more accurate distinction between true neurotoxins and cytotoxins in an in-vitro testing environment.
An early example of neurotoxin based targeting used radiolabeled tetrodotoxin to assay sodium channels and obtain precise measurements about their concentration along nerve membranes.
Common symptoms of TTX consumption include paraesthesia (often restricted to the mouth and limbs), muscle weakness, nausea, and vomiting[55] and often manifest within 30 minutes of ingestion.
[6] The effect of this increased signaling threshold is a reduced excitability of postsynaptic neurons, and subsequent loss of motor and sensory function which can result in paralysis and death.
[55] Tetraethylammonium (TEA) is a compound that, like a number of neurotoxins, was first identified through its damaging effects to the nervous system and shown to have the capacity of inhibiting the function of motor nerves and thus the contraction of the musculature in a manner similar to that of curare.
Evidence has shown that Cltx can inhibit the ability for gliomas to infiltrate healthy nervous tissue in the brain, significantly reducing the potential invasive harm caused by tumors.
Significantly, ω-CgTx is capable of long term binding to and inhibition of voltage-dependent calcium channels located in the membranes of neurons but not those of muscle cells.
[67] A notably unique feature of BTX is its relatively common therapeutic use in treating dystonia and spasticity disorders,[67] as well as in inducing muscular atrophy[11] despite being the most poisonous substance known.
[35] As the toxin is highly biologically active, an estimated dose of 1μg/kg body weight is sufficient to induce an insufficient tidal volume and resultant death by asphyxiation.
It has been shown that capsaicin (active compound responsible for heat in chili peppers) can bind the TRPV1 receptor expressed on cholinergic neurons and inhibit the toxic effects of BTX.
[37] A loss of function in the BBB can produce significant damage to the neurons in the CNS, as the barrier protecting the brain from other toxins found in the blood will no longer be capable of such action.
[80] There is continued interest in anatoxin-a because of the dangers it presents to recreational and drinking waters, and because it is a particularly useful molecule for investigating acetylcholine receptors in the nervous system.
[82] Bungarotoxin is a compound with known interaction with nicotinic acetylcholine receptors (nAChRs), which constitute a family of ion channels whose activity is triggered by neurotransmitter binding.
[39] Though extremely toxic if ingested, α-bungarotoxin has shown extensive usefulness in neuroscience as it is particularly adept at isolating nAChRs due to its high affinity to the receptors.
[84] This α7-nAChR functions to allow calcium ion influx into cells, and thus when blocked by ingested bungarotoxin will produce damaging effects, as ACh signaling will be inhibited.
By blocking the receptors, the neurotoxin is capable of significantly reducing neuromuscular junction signaling, an effect which has resulted in its use by anesthesiologists to produce muscular relaxation.
The mechanism of this cytotoxicity functions through arsenite-induced increases in intracellular calcium ion levels within neurons, which may subsequently reduce mitochondrial transmembrane potential which activates caspases, triggering cell death.
[97] An underlying mechanism by which lead is able to cause harm is its ability to be transported by calcium ATPase pumps across the BBB, allowing for direct contact with the fragile cells within the central nervous system.
[48] It is this intracellular calcium increase that activates protein kinase C (PKC), which manifests as learning deficits in children as a result of early lead exposure.
[104] With chronic ethanol intake, however, the susceptibility of these NMDA receptors to induce LTP increases in the mesolimbic dopamine neurons in an inositol 1,4,5-triphosphate (IP3) dependent manner.
[111][112][113][51] MPP+, the toxic metabolite of MPTP is a selective neurotoxin which interferes with oxidative phosphorylation in mitochondria by inhibiting complex I, leading to the depletion of ATP and subsequent cell death.
[9] Though nitric oxide (NO) is commonly used by the nervous system in inter-neuron communication and signaling, it can be active in mechanisms leading to ischemia in the cerebrum (Iadecola 1998).
Glutamate, like nitric oxide, is an endogenously produced compound used by neurons to perform normally, being present in small concentrations throughout the gray matter of the CNS.