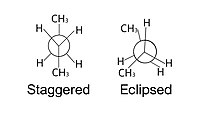
Newman projection
[1] This projection most commonly sights down a carbon-carbon bond, making it a very useful way to visualize the stereochemistry of alkanes.
This type of representation clearly illustrates the specific dihedral angle between the proximal and distal atoms.
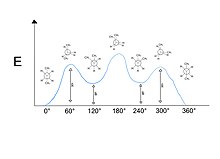
[5] Anti interactions refer to the molecules (usually of the same type) sitting exactly opposite of each other at 180° on the Newman projection.
In reality, these species are in line with each other, but are drawn slightly staggered to help format the projection onto paper.
These types of conformations are generally higher in energy due to increased bond strain.