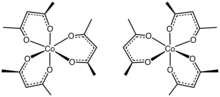
Tris(acetylacetonato)cobalt(III)
Tris(acetylacetonato)cobalt(III) is the coordination complex with the formula Co(C5H7O2)3.
Often abbreviated Co(acac)3, it is a green, diamagnetic solid that is soluble in organic solvents, but not in water.
Owing to its solubility in organic solvents, tris(acetylacetonato)cobalt(III) is used to produce homogeneous catalysts by reduction.
The three acac− ligands bind in a bidentate fashion to cobalt, defining an octahedral complex.
Tris(acetylacetonato)cobalt(III) is prepared by the reaction of cobalt(II) carbonate and acetylacetone in the presence of hydrogen peroxide:[3] One distinctive aspect of Co(acac)3 is its susceptibility toward electrophilic aromatic substitution, by which protons on the central carbon are replaced with diverse electrophiles (Me = methyl):[4]