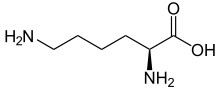
Lysine
Lysine plays several roles in humans, most importantly proteinogenesis, but also in the crosslinking of collagen polypeptides, uptake of essential mineral nutrients, and in the production of carnitine, which is key in fatty acid metabolism.
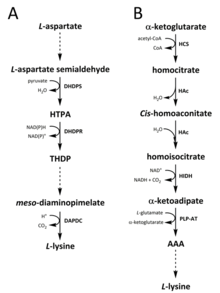
[11][12] The DAP pathway is found in both prokaryotes and plants and begins with the dihydrodipicolinate synthase (DHDPS) (E.C 4.3.3.7) catalysed condensation reaction between the aspartate derived, L-aspartate semialdehyde, and pyruvate to form (4S)-4-hydroxy-2,3,4,5-tetrahydro-(2S)-dipicolinic acid (HTPA).
[20] These four variant pathways converge at the formation of the penultimate product, meso‑diaminopimelate, which is subsequently enzymatically decarboxylated in an irreversible reaction catalysed by diaminopimelate decarboxylase (DAPDC) (E.C 4.1.1.20) to produce L-lysine.
[21][22] The DAP pathway is regulated at multiple levels, including upstream at the enzymes involved in aspartate processing as well as at the initial DHDPS catalysed condensation step.
[12][24][25][26][27][28][29] It has also been reported that an alternative variant of the AAA route has been found in Thermus thermophilus and Pyrococcus horikoshii, which could indicate that this pathway is more widely spread in prokaryotes than originally proposed.
[30][31][32] The first and rate-limiting step in the AAA pathway is the condensation reaction between acetyl-CoA and α‑ketoglutarate catalysed by homocitrate-synthase (HCS) (E.C 2.3.3.14) to give the intermediate homocitryl‑CoA, which is hydrolysed by the same enzyme to produce homocitrate.
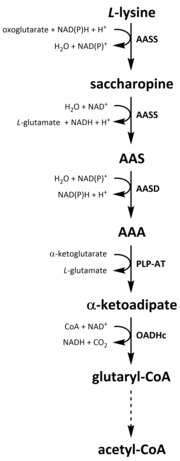
[40][41] The first step involves the LKR catalysed reduction of L-lysine in the presence of α-ketoglutarate to produce saccharopine, with NAD(P)H acting as a proton donor.
[51][52] Often these practices have involved the intentional dysregulation of the DAP pathway by means of introducing lysine feedback-insensitive orthologues of the DHDPS enzyme.
[55][56] While genetic modification practices have met limited success, more traditional selective breeding techniques have allowed for the isolation of "Quality Protein Maize", which has significantly increased levels of lysine and tryptophan, also an essential amino acid.
Since its side chain contains a positively charged group on one end and a long hydrophobic carbon tail close to the backbone, lysine is considered somewhat amphipathic.
For this reason, lysine can be found buried as well as more commonly in solvent channels and on the exterior of proteins, where it can interact with the aqueous environment.
[65] Lysine can also contribute to protein stability as its ε-amino group often participates in hydrogen bonding, salt bridges and covalent interactions to form a Schiff base.
Modifications often include the addition or removal of an acetyl (−CH3CO) forming acetyllysine or reverting to lysine, up to three methyl (−CH3), ubiquitin or a sumo protein group.
Lysine has also been implicated to play a key role in other biological processes including; structural proteins of connective tissues, calcium homeostasis, and fatty acid metabolism.
[75][76][77] Lysine has been shown to be involved in the crosslinking between the three helical polypeptides in collagen, resulting in its stability and tensile strength.
[79] This concept has previously been explored as a means to circumvent the unwanted release of potentially pathogenic genetically modified bacteria.
[76] Finally, lysine has been shown to be a precursor for carnitine, which transports fatty acids to the mitochondria, where they can be oxidised for the release of energy.
There has been a long discussion that lysine, when administered intravenously or orally, can significantly increase the release of growth hormones.
[90] Most commonly, lysine deficiency is seen in non-western societies and manifests as protein-energy malnutrition, which has profound and systemic effects on the health of the individual.
[93] Due to a lack of lysine catabolism, the amino acid accumulates in plasma and patients develop hyperlysinaemia, which can present as asymptomatic to severe neurological disabilities, including epilepsy, ataxia, spasticity, and psychomotor impairment.
[101] Lysine production for animal feed is a major global industry, reaching in 2009 almost 700,000 tons for a market value of over €1.22 billion.
Lysine supplementation allows for the use of lower-cost plant protein (maize, for instance, rather than soy) while maintaining high growth rates, and limiting the pollution from nitrogen excretion.
Genetic engineering research is actively pursuing bacterial strains to improve the efficiency of production and allow lysine to be made from other substrates.
[102] The most common bacteria used is Corynebacterium glutamicum specially mutagenized or gene-engineered to produce lysine, but analogous strains of Escherichia coli are also employed.
The Archer Daniels Midland Company paid a fine of US$100 million, and three of its executives were convicted and served prison time.
In the 2009 episode of the American sitcom, The Big Bang Theory, entitled The Friendship Algorithm, Sheldon is trying to befriend his work nemesis, Barry Kripke, to get time on some equipment he needs for an experiment.
[109][110] This article was adapted from the following source under a CC BY 4.0 license (2018) (reviewer reports): Cody J Hall; Tatiana P. Soares da Costa (1 June 2018).