Nomenclature of monoclonal antibodies
An antibody is a protein that is produced in B cells and used by the immune system of humans and other vertebrate animals to identify a specific foreign object like a bacterium or a virus.
Monoclonal antibodies are those that were produced in identical cells, often artificially, and so share the same target object.
Unlike most other pharmaceuticals, monoclonal antibody nomenclature uses different preceding word parts (morphemes) depending on structure and function.
Group 2 has the stem -bart for full-length antibodies artificial, which contain one or more engineered regions (at least one point mutation).
[7] The first monoclonal antibodies were produced in mice (substem -o-, yielding the ending -omab; usually Mus musculus, the house mouse) or other non-human organisms.
[9] These non-human antibodies are recognized as foreign by the human immune system and may be rapidly cleared from the body, provoke an allergic reaction, or both.
If the constant region is replaced with the human form, the antibody is termed chimeric and the substem used was -xi-.
-mul-, for example, is never reduced to -mu- because no chimeric or humanized antibodies targeting the musculoskeletal system ever received an INN.
[29] The principle of monoclonal antibody production, called hybridoma technology, was published in 1975 by Georges Köhler and César Milstein,[30] who were awarded the 1984 Medicine Nobel Prize for their discovery together with Niels Kaj Jerne.
[32] The World Health Organization (WHO) introduced the system of International Nonproprietary Names in 1950, with the first INN list being published three years later.
The stem -mab for monoclonal antibodies was proposed around 1990, and the current system with target and source substems was developed between 1991 and 1993.
Due to the collaboration between the WHO and the United States Adopted Names Council, antibody USANs have the same structure and are largely identical to INNs.
[9][33] In October 2008, the WHO convoked a working group to revise the nomenclature of monoclonal antibodies, to meet challenges discussed in April the same year.
[23] The difficulty in capturing the complexity and subtleties of the many methods by which antibody drugs can be produced is one of the reasons that the INN dropped the source substem, as is the need for creating more clearly distinguishable names.
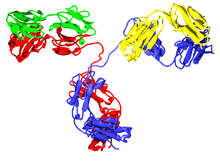

Human parts are shown in brown, non-human parts in blue. The variable domains are the boxes on top of each antibody; the CDRs within these domains are represented as triple loops.
