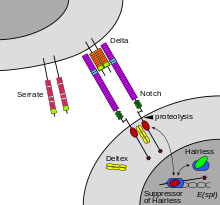
Notch signaling pathway
[18] Compelling evidence for this model was provided in 1998 by in vivo analysis in Drosophila by Gary Struhl[19] and in cell culture by Raphael Kopan.
lin-12 and Notch mediate binary cell fate decisions, and lateral inhibition involves feedback mechanisms to amplify initial differences.
Binding of ligand promotes two proteolytic processing events; as a result of proteolysis, the intracellular domain is liberated and can enter the nucleus to engage other DNA-binding proteins and regulate gene expression.
[citation needed] The notch extracellular domain is composed primarily of small cystine-rich motifs called EGF-like repeats.
This releases the intracellular domain of the notch protein (NICD), which then moves to the nucleus, where it can regulate gene expression by activating the transcription factor CSL.
[42][43] The Notch signaling pathway is important for cell-cell communication, which involves gene regulation mechanisms that control multiple cell differentiation processes during embryonic and adult life.
[60] Early studies in the nematode model organism C. elegans indicate that Notch signaling has a major role in the induction of mesoderm and cell fate determination.
[64][65][66] Experiments with Delta1 mutant mice that show abnormal somitogenesis with loss of anterior/posterior polarity suggest that Notch signaling is also necessary for the maintenance of somite borders.
This process is highly regulated as somites must have the correct size and spacing in order to avoid malformations within the axial skeleton that may potentially lead to spondylocostal dysostosis.
[67] Notch signaling is known to occur inside ciliated, differentiating cells found in the first epidermal layers during early skin development.
Early findings on Notch signaling in central nervous system (CNS) development were performed mainly in Drosophila with mutagenesis experiments.
In the past decade, advances in mutation and knockout techniques allowed research on the Notch signaling pathway in mammalian models, especially rodents.
In recent years, other functions of the Notch pathway have also been found, including glial cell specification,[71][72] neurites development,[73] as well as learning and memory.
[85] Several gamma secretase inhibitors that underwent human clinical trials in Alzheimer's disease and MCI patients resulted in statistically significant worsening of cognition relative to controls, which is thought to be due to its incidental effect on Notch signalling.
[88] Some studies in Xenopus[94] and in mouse embryonic stem cells[95] indicate that cardiomyogenic commitment and differentiation require Notch signaling inhibition.
Active Notch signaling is required in the ventricular endocardium for proper trabeculae development subsequent to myocardial specification by regulating BMP10, NRG1, and EphrinB2 expression.
[100] The downstream effector of Notch signaling, HEY2, was also demonstrated to be important in regulating ventricular development by its expression in the interventricular septum and the endocardial cells of the cardiac cushions.
[101] Cardiomyocyte and smooth muscle cell-specific deletion of HEY2 results in impaired cardiac contractility, malformed right ventricle, and ventricular septal defects.
Likewise, during the sprouting process itself, the migratory behavior of connector cells must be limited to retain a patent connection to the original blood vessel.
[112][113] Evidence suggests Notch signaling regulates the progressive recruitment of endocrine cell types from a common precursor,[114] acting through two possible mechanisms.
Mutations in elements of the Notch signaling pathway affect the earliest intestinal cell fate decisions during zebrafish development.
Overall, Notch signaling has a major role in the commitment of mesenchymal cells to the osteoblastic lineage and provides a possible therapeutic approach to bone regeneration.
[124][125] Additionally, in ventricular cardiomyocytes, which stop dividing shortly after birth, NOTCH2 signaling activation promotes cell cycle reentry.
[132] In hepatocellular carcinoma, for instance, it was suggesting that AXIN1 mutations would provoke Notch signaling pathway activation, fostering the cancer development, but a recent study demonstrated that such an effect cannot be detected.
[136] Preclinical studies showed beneficial effects of gamma-secretase inhibitors in endometriosis,[137] a disease characterised by increased expression of notch pathway constituents.
[140][141] Mathematical modeling in Notch-Delta signaling has become a pivotal tool in understanding pattern formation driven by cell-cell interactions, particularly in the context of lateral-inhibition mechanisms.
The Collier model,[142] a cornerstone in this field, employs a system of coupled ordinary differential equations to describe the feedback loop between adjacent cells.
The application of mathematical modeling in Notch-Delta signaling has been particularly illuminating in understanding the patterning of sensory organ precursors (SOPs) in the Drosophila's notum and wing margin.
[147][148] The mathematical modeling of Notch-Delta signaling thus provides significant insights into lateral inhibition mechanisms and pattern formation in biological systems.
It enhances the understanding of cell-cell interaction variations leading to diverse tissue structures, contributing to developmental biology and offering potential therapeutic pathways in diseases related to Notch-Delta dysregulation.