Potassium octachlorodimolybdate
Potassium octachlorodimolybdate (systematically named potassium bis(tetrachloromolybdate)(Mo–Mo)(4−)) is an inorganic compound with the chemical formula K4[Mo2Cl8].
The anion is of historic interest as one of the earliest illustrations of a quadruple bonding.
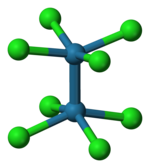
The compound is prepared in two steps from molybdenum hexacarbonyl:[1][2] The reaction of the acetate with HCl was first described as providing trimolybdenum compounds,[3] but subsequent crystallographic analysis confirmed that the salt contains the [Cl4Mo≣MoCl4]4− anion, with D4h symmetry, in which the two Mo atoms are linked by a quadruple bond.
Each Mo atom is bounded with four Cl− ligands by a single bond.
Each MoCl4 group is a regular square pyramid, with an Mo atom at the apex, and four Cl atoms at the vertices of the square base of the pyramid.