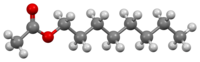
Octyl acetate
Octyl acetate, or octyl ethanoate, is an organic compound with the formula CH3(CH2)7O2CCH3.
It is classified as an ester that is formed from 1-octanol (octyl alcohol) and acetic acid.
It is found in oranges, grapefruits, and other citrus products.
[10] Octyl acetate can be synthesized by the Fischer esterification of 1-octanol and acetic acid: Because of its fruity odor,[11] octyl acetate is used as the basis for artificial flavors and in perfumery.
It is also a solvent for nitrocellulose, waxes, oils, and some resins.