Oil dispersant
Alaska had fewer than 4,000 gallons of dispersants available at the time of the Exxon Valdez oil spill, and no aircraft with which to dispense them.
The dispersants introduced were relatively ineffective due to insufficient wave action to mix the oil and water, and their use was shortly abandoned.
[6] A report by David Kirby for TakePart found that the main component of the Corexit 9527 formulation used during Exxon Valdez cleanup, 2-butoxyethanol, was identified as "one of the agents that caused liver, kidney, lung, nervous system, and blood disorders among cleanup crews in Alaska following the 1989 Exxon Valdez spill.
[14] In 2013, in response to the growing body of laboratory-derived toxicity data, some researchers address the scrutiny that should be used when evaluating laboratory test results that have been extrapolated using procedures that are not fully reliable for environmental assessments.
[15][16] Since then, guidance has been published that improves the comparability and relevance of oil toxicity tests.
[17] Maritime New Zealand used the oil dispersant Corexit 9500 to help in the cleanup process.
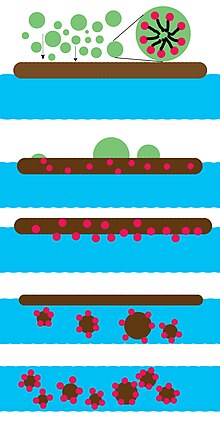
[19] Surfactants reduce oil-water interfacial tension, which helps waves break oil into small droplets.
The salinity of the water is more important for ionic-surfactant dispersants, as salt screens electrostatic interactions between molecules.
[23] There are several parameters which must be considered when creating a dispersion model, including oil-slick thickness, advection, resurfacing and wave action.
[23] A general problem in modeling dispersants is that they change several of these parameters; surfactants lower the thickness of the film, increase the amount of diffusion into the water column and increase the amount of breakup caused by wave action.
This causes the oil slick's behavior to be more dominated by vertical diffusion than horizontal advection.
where Mackay's model predicts an increasing dispersion rate, as the slick becomes thinner in one dimension.
It considers particles to be in one of three states: at the surface, entrained in the water column or evaporated.
The empirically based model uses probabilistic variables to determine where the dispersant will move and where it will go after it breaks up oil slicks.
Surfactants are classified into four main types, each with different properties and applications: anionic, cationic, nonionic and zwitterionic (or amphoteric).
An example of a zwitterionic compound is phosphatidylcholine, which as a lipid is largely insoluble in water.
[26] Surfactant behavior is highly dependent on the hydrophilic-lipophilic balance (HLB) value.
Two formulations of different dispersing agents for oil spills, Dispersit and Omni-Clean, are shown below.
Concerns regarding the persistence in the environment and toxicity to various flora and fauna of oil dispersants date back to their early use in the 1960s and 1970s.
Sufficient dispersant with droplets in the proper size are necessary; this can be achieved with an appropriate pumping rate.