Surfactant
[2] As they consist of a water-repellent and a water-attracting part, they enable water and oil to mix; they can form foam and facilitate the detachment of dirt.
Surfactants occur naturally in traditional plant-based detergents, e.g. horse chestnuts or soap nuts; they can also be found in the secretions of some caterpillars.
However, surfactants are increasingly produced in whole or in part from renewable biomass, like sugar, fatty alcohol from vegetable oils, by-products of biofuel production, or other biogenic material.
The polyether groups often comprise ethoxylated (polyethylene oxide-like) sequences inserted to increase the hydrophilic character of a surfactant.
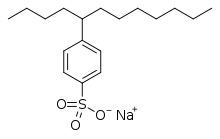
More specialized species include sodium lauroyl sarcosinate and carboxylate-based fluorosurfactants such as perfluorononanoate, perfluorooctanoate (PFOA or PFO).
The most common biological zwitterionic surfactants have a phosphate anion with an amine or ammonium, such as the phospholipids phosphatidylserine, phosphatidylethanolamine, phosphatidylcholine, and sphingomyelins.
The differences between the individual types of non-ionic surfactants are slight, and the choice is primarily governed having regard to the costs of special properties (e.g., effectiveness and efficiency, toxicity, dermatological compatibility, biodegradability) or permission for use in food.
[6] Fatty acid ethoxylates are a class of very versatile surfactants, which combine in a single molecule the characteristic of a weakly anionic, pH-responsive head group with the presence of stabilizing and temperature responsive ethyleneoxide units.
The shape of the aggregates depends on the chemical structure of the surfactants, namely the balance in size between the hydrophilic head and hydrophobic tail.
The dynamics of surfactant adsorption is of great importance for practical applications such as in foaming, emulsifying or coating processes, where bubbles or drops are rapidly generated and need to be stabilized.
Surfactants play an important role as cleaning, wetting, dispersing, emulsifying, foaming and anti-foaming agents in many practical applications and products, including detergents, fabric softeners, motor oils, emulsions, soaps, paints, adhesives, inks, anti-fogs, ski waxes, snowboard wax, deinking of recycled papers, in flotation, washing and enzymatic processes, and laxatives.
Surfactants are used in firefighting (to make "wet water" that more quickly soaks into flammable materials[11][12]) and pipelines (liquid drag reducing agents).
Surfactants act to cause the displacement of air from the matrix of cotton pads and bandages so that medicinal solutions can be absorbed for application to various body areas.
They also act to displace dirt and debris by the use of detergents in the washing of wounds[13] and via the application of medicinal lotions and sprays to surface of skin and mucous membranes.
[15] In solution, detergents help solubilize a variety of chemical species by dissociating aggregates and unfolding proteins.
Popular surfactants in the biochemistry laboratory are sodium lauryl sulfate (SDS) and cetyl trimethylammonium bromide (CTAB).
Surfactants are used with quantum dots in order to manipulate their growth,[17] assembly, and electrical properties, in addition to mediating reactions on their surfaces.
One example of a pharmaceutical pulmonary surfactant is Survanta (beractant) or its generic form Beraksurf, produced by Abbvie and Tekzima respectively.
[6] Surfactants are routinely deposited in numerous ways on land and into water systems, whether as part of an intended process or as industrial and household waste.
[22][23][24] Anionic surfactants can be found in soils as the result of sewage sludge application, wastewater irrigation, and remediation processes.
[25][26] In the case of the Deepwater Horizon oil spill, unprecedented amounts of Corexit were sprayed directly into the ocean at the leak and on the sea-water's surface.
[30][31] Two major surfactants, linear alkylbenzene sulfonates (LAS) and the alkyl phenol ethoxylates (APE) break down under aerobic conditions found in sewage treatment plants and in soil to nonylphenol, which is thought to be an endocrine disruptor.