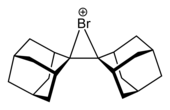
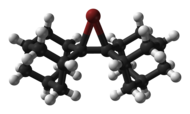
Halonium ion
[1] The 3-membered cyclic variety commonly proposed as intermediates in electrophilic halogenation may be called haliranium ions, using the Hantzsch-Widman nomenclature system.
[2] Diaryliodonium ions ([Ar2I]+X−) are generally stable, isolable salts which exhibit a T-shaped geometry with the aryl groups at ~90 degrees apart;[3] for more details, see hypervalent iodine.
[4] Halonium ions were first postulated in 1937 by Roberts and Kimball[5] to account for observed anti diastereoselectivity in halogen addition reactions to alkenes.
They also asserted that a positively charged halogen atom is isoelectronic with oxygen and that carbon and bromine have comparable ionization potentials.
For certain aryl substituted alkenes, the anti stereospecificity is diminished or lost, as a result of weakened or absent halonium character in the cationic intermediate.
A fluoronium ion was recently characterized in solution phase (dissolved in sulfur dioxide or sulfuryl chloride fluoride) at low temperature.