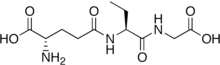
Ophthalmic acid
However, instead of the cysteine essential for many of glutathione's diverse functions, it contains L-2-aminobutyrate, a non-proteinogenic amino acid lacking the nucleophilic thiol group.
In 2024, an article published by the federation of European biochemistry societies compiled evidence to put forward the major hypothesis that OPH serves as a glutathione regulating tripeptide, affecting both cellular and organelle influx and efflux of GSH, as well as modulating GSH-dependent reactions and signaling.
Major regulators of OPH biosynthesis are local (relative) concentrations of cysteine and 2-aminobutyric acid, as well as their γ-glutamyl intermediate products.
[2] OPH was first discovered and isolated from calf lens[3] in 1956, and has since been found to be a ubiquitous metabolite.
It is produced by: Distribution within (higher) organisms also appears to be ubiquitous as it has been found in the: In plants, it is found in: OPH has mostly appeared in metabolomics studies correlating changes in its abundance with oxidative stress, following a study from 2006 on acetaminophen overdose in mice.