Orcinol
Orcinol has been detected in the "toxic glue" of the ant species Camponotus saundersi.
Orcinol was first prepared by dehydroacetic acid, a conversion that involved ring-opening of the pyrone to a triketone.
It may be synthesized from toluene; more interesting is its production when acetone dicarboxylic ester is condensed with the aid of sodium.
Oxidation of the ammoniacal solution gives orcein, C28H24N2O7, the chief constituent of the natural dye archil.
[2] It is the main water-soluble phenol in the oil, and has been extracted and refined industrially by Viru Keemia Grupp.
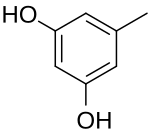

Orcinol