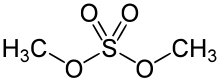
Dimethyl sulfate
Compared to other methylating agents, dimethyl sulfate is preferred by the industry because of its low cost and high reactivity.
Dimethyl sulfate can effect the base-specific cleavage of DNA by attacking the imidazole rings present in guanine.
Although dimethyl sulfate is highly effective and affordable, its toxicity has encouraged the use of other methylating reagents.
Dimethyl sulfate is carcinogenic[19] and mutagenic, highly poisonous, corrosive, and environmentally hazardous.
[21] The vapor pressure of 65 Pa[22] is sufficiently large to produce a lethal concentration in air by evaporation at 20 °C.
Delayed toxicity allows potentially fatal exposures to occur prior to development of any warning symptoms.
[20] Symptoms may be delayed 6–24 h. Concentrated solutions of bases (ammonia, alkalis) can be used to hydrolyze minor spills and residues on contaminated equipment, but the reaction may become violent with larger amounts of dimethyl sulfate (see ICSC).
One hypothesis regarding the apparently mysterious 1994 "toxic lady" incident is that the person at the centre of the incident had built up dimethyl sulfone crystals in her blood, which were converted by an unknown mechanism to dimethyl sulfate vapour that poisoned attending medical staff.