Organogels
In polymer chemistry, an organogel is a class of gel composed of an organic liquid phase within a three-dimensional, cross-linked network.
This mechanism converts a precursor solution of monomers with various reactive sites into polymeric chains that grow into a single covalently-linked network.
At a critical concentration (the gel point), the polymeric network becomes large enough so that on the macroscopic scale, the solution starts to exhibit gel-like physical properties: an extensive continuous solid network, no steady-state flow, and solid-like rheological properties.
The formulation of an accurate theory of gel formation that correctly predicts gelation parameters (such as time, rate, and structure) of a broad range of materials is highly sought after for both commercial and intellectual reasons.
As of 2014[update] most researchers used statistical methods, as the equations derived thereby are less cumbersome and contain variables to which specific physical meanings can be attached, thus aiding in the analysis of gel formation theory.
This is due in large part to small increases in accuracy provided by the use of more complicated methods, and to its being a general model which can be applied to many gelation systems.
In general, kinetic treatments of gelation result in large, unwieldy, and dense sets of equations that give answers not discernibly better than those given by the statistical approach.
[7],[8],[9] The statistical approach views the phase change from liquid to gel as a uniform process throughout the fluid.
Statistical theories try to determine the fraction of the total possible bonds that need to be made before an infinite polymer network can appear.
[10],[11] Using the above assumptions, let us examine a homopolymerization reaction starting from a single monomer with z-functional groups with a fraction p of all possible bonds already having been formed.
The polymer we create follows the form of a Cayley tree or Bethe lattice – known from the field of statistical mechanics.
As we follow the tree's branches we want there to always be at least one path that leads onwards, as this is the condition of an infinite network polymer.
So a pc of 1/2 means that the first point in time that an infinite network will be able to exist will be when 1/2 of all possible bonds have been made by the monomers.
This equation is derived for the simple case of a self-reacting monomer with a single type of reacting group A.
If the starting concentrations of A and B reactive sites are the same, then pApB can be condensed to pgel2 and values for the fraction of all bonds at which an infinite network will form can be found.
Generalisation of these results to monomers with multiple types of functional groups is obtained with Random graph theory of gelation.
Molecular self-assembly is a process by which molecules adopt a defined arrangement without guidance or management from an external source.
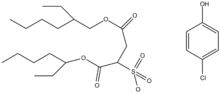
[22] Garner et al.[15] explored the importance of organogelator structures using 4-tertbutyl-1-aryl cy-clohexanol derivatives showing that a phenyl group in an axial configuration induces gelation, unlike derivatives with the phenyl group in equatorial configuration.
[15] Polymeric organogelators can induce gelation even at very low concentrations (less than 20 g/L) and the self-assembly capability could be customized by modifying the chemical structure of the polymer backbone.
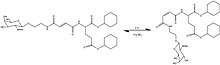
Matsumoto et al.[24] used UV light to trigger trans–cis photoisomerization of fumaric amide units causing self-assembly or disassembly to a gel or the corresponding sol, respectively (See Figure 2).
Chen et al.[19] designed a system that would undergo self-assembly by triggering changes in intermolecular interactions.
NO has been used as an analyte or biomarker for disease detection, and the discovery of NO's role in analyte-triggered gelation system no doubt has opened new doors to the world of chemical sensing.
[27] Use of SEM can distinguish between gels that have a fibrous network as opposed to those that have a three-dimensional cross linked structure.
[28] This technique utilizes a similar approach when compared to ball indentation only on a significantly small scale.
The viscoelastic properties of gels mean that they undergo time dependent structural changes in response to a physical deformation.
In both techniques, a damping mechanism is resolved with both differing frequency and time in order to determine the viscoelastic properties of the material.