Osmium(IV) chloride
It exists in two polymorphs (crystalline forms).
The compound is used to prepare other osmium complexes.
It was first reported in 1909 as the product of chlorination of osmium metal.
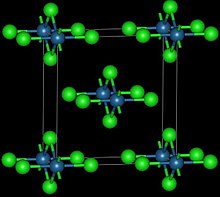
[1] This route affords the high temperature polymorph:[2] This reddish-black polymorph is orthorhombic and adopts a structure in which osmium centres are octahedrally coordinated, sharing opposite edges of the OsCl6 octahedra to form a chain.
[3] A brown, apparently cubic polymorph forms upon reduction of osmium tetroxide with thionyl chloride:[4] Osmium tetraoxide dissolves in hydrochloric acid to give the hexachloroosmate anion: