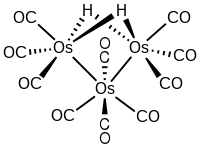
Decacarbonyldihydridotriosmium
This purple-violet crystalline air-stable cluster is noteworthy because it is electron-deficient and hence adds a variety of substrates.
The trinuclear cluster features an isosceles triangular array of metals with one short edge (rOs-Os = 2.68 Å), which is spanned by the two hydride ligands, and two longer edges (rOs-Os = 2.81 Å).
[2] It is prepared by purging a solution of Os3(CO)12 in octane (or other inert solvent of similar boiling point) with H2.
[3] The cluster reacts with a wide range of reagents under mild conditions.
Illustrative is its reaction with diazomethane to give Os3(CO)10(μ-H)(μ-CH3), exhibiting an agostic interaction, the first identified in a metal cluster.