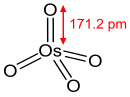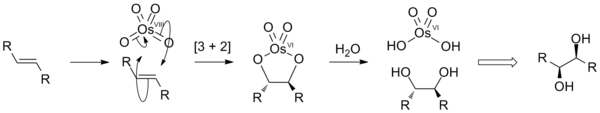Osmium tetroxide
The osmium of OsO4 has an oxidation number of VIII; however, the metal does not possess a corresponding 8+ charge as the bonding in the compound is largely covalent in character (the ionization energy required to produce a formal 8+ charge also far exceeds the energies available in normal chemical reactions).
The osmium atom exhibits double bonds to the four oxide ligands, resulting in a 16 electron complex.
This proceeds via a [3 + 2] cycloaddition reaction between the OsO4 and alkene to form an intermediate osmate ester that rapidly hydrolyses to yield the vicinal diol.
Typical reagents include H2O2 (Milas hydroxylation), N-methylmorpholine N-oxide (Upjohn dihydroxylation) and K3Fe(CN)6/water.
[15] The process can be extended to give two aldehydes in the Lemieux–Johnson oxidation, which uses periodate to achieve diol cleavage and to regenerate the catalytic loading of OsO4.
[18] With tert-BuNH2, the imido derivative is produced: Similarly, with NH3 one obtains the nitrido complex: The [Os(N)O3]− anion is isoelectronic and isostructural with OsO4.
The suspended osmium metal can be used to catalytically hydrogenate a wide variety of organic chemicals containing double or triple bonds.
OsO4 undergoes "reductive carbonylation" with carbon monoxide in methanol at 400 K and 200 sbar to produce the triangular cluster Os3(CO)12: Osmium forms several oxofluorides, all of which are very sensitive to moisture.
Purple cis-OsO2F4 forms at 77 K in an anhydrous HF solution:[19] OsO4 also reacts with F2 to form yellow OsO3F2:[20] OsO4 reacts with one equivalent of [Me4N]F at 298 K and 2 equivalents at 253 K:[12] In organic synthesis OsO4 is widely used to oxidize alkenes to the vicinal diols, adding two hydroxyl groups at the same side (syn addition).
Osmium(VIII) oxide is also used in catalytic amounts in the Sharpless oxyamination to give vicinal amino-alcohols.
OsO4 is a widely used staining agent used in transmission electron microscopy (TEM) to provide contrast to the image.
In the staining of the plasma membrane, osmium(VIII) oxide binds phospholipid head regions, thus creating contrast with the neighbouring protoplasm (cytoplasm).
The presence of a heavy metal is sufficient to block the electron beam, so the polystyrene domains are seen clearly in thin films in TEM.
In the final stages of refining, crude OsO4 is dissolved in alcoholic NaOH forming Na2[OsO2(OH)4], which, when treated with NH4Cl, to give (NH4)4[OsO2Cl2].
The adduct broke the fullerene's symmetry, allowing for crystallization and confirmation of the structure of C60 by X-ray crystallography.
[8] Osmium(VIII) oxide can penetrate plastics and food packaging, and therefore must be stored in glass under refrigeration.