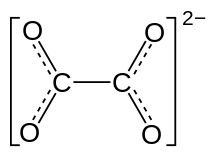
Oxalate
The loss of the second proton, which yields the oxalate ion, has an equilibrium constant of 5.25×10−5 (pKa = 4.28).
[citation needed] The oxalate anion exists in a nonplanar conformation where the O–C–C–O dihedrals approach 90° with approximate D2d symmetry.
Several plant foods such as the root and/or leaves of spinach, rhubarb, and buckwheat are high in oxalic acid and can contribute to the formation of kidney stones in some individuals.
[14] Other edible plants with significant concentrations of oxalate include, in decreasing order, star fruit (carambola), black pepper, parsley, poppy seed, amaranth, chard, beets, cocoa, chocolate, most nuts, most berries, fishtail palms, New Zealand spinach (Tetragonia tetragonioides), and beans.
However, the drink derived by infusion in hot water typically contains only low to moderate amounts of oxalic acid due to the small mass of leaves used for brewing.
Although unusual, consumption of oxalates (for example, the grazing of animals on oxalate-containing plants such as Bassia hyssopifolia, or human consumption of wood sorrel or, specifically in excessive quantities, black tea) may result in kidney disease or even death due to oxalate poisoning.
The New England Journal of Medicine reported acute oxalate nephropathy "almost certainly due to excessive consumption of iced tea" in a 56-year-old man, who drank "sixteen 8-ounce glasses of iced tea daily" (roughly 3.8 liters).
The authors of the paper hypothesized that acute oxalate nephropathy is an underdiagnosed cause of kidney failure and suggested thorough examination of patient dietary history in cases of unexplained kidney failure without proteinuria (an excess of protein in the urine) and with large amounts of calcium oxalate in urine sediment.