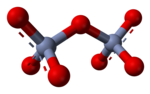
Oxyanion
The structures of condensed oxyanions can be rationalized in terms of AOn polyhedral units with sharing of corners or edges between polyhedra.
The formula of monomeric oxyanions, AOm−n, is dictated by the oxidation state of the element A and its position in the periodic table.
Many oxyanions of elements in lower oxidation state obey the octet rule and this can be used to rationalize the formulae adopted.
In the third and subsequent rows of the periodic table, 6-coordination is possible, but isolated octahedral oxyanions are not known because they would carry too high an electrical charge.
Fully protonated oxyanions with an octahedral structure are found in such species as Sn(OH)2−6 and Sb(OH)−6.
In addition, orthoperiodate can be only partially deprotonated,[Note 1] with The naming of monomeric oxyanions follows the following rules.
In aqueous solution, oxyanions with high charge can undergo condensation reactions, such as in the formation of the dichromate ion, Cr2O2−7: The driving force for this reaction is the reduction of electrical charge density on the anion and the elimination of the hydronium (H+) ion.
The conversion of ATP to ADP is a hydrolysis reaction and is an important source of energy in biological systems.
This results in an ideal formula Si4O6−11 and a linear chain structure which explains the fibrous nature of these minerals.
Sharing of all three corners can result in a sheet structure, as in mica, Si2O2−5, in which each silicon has one oxygen to itself and a half-share in three others.
However, the oxidation state of aluminium is one less than that of silicon, so the replacement must be accompanied by the addition of another cation.
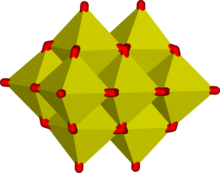
[5][6] Edge-sharing is common in ions containing octahedral building blocks and the octahedra are usually distorted to reduce the strain at the bridging oxygen atoms.
The efficacy of edge-sharing is demonstrated by the following reaction, which occurs when an alkaline aqueous solution of molybdate is acidified.
The tetrahedral molybdate ion is converted into a cluster of 7 edge-linked octahedra[6][7] giving an average charge on each molybdenum of 6⁄7.
The extent of protonation in aqueous solution will depend on the acid dissociation constants and pH.
Predominance diagrams can become very complicated when many polymeric species can be formed,[10] such as in vanadates, molybdates, and tungstates.