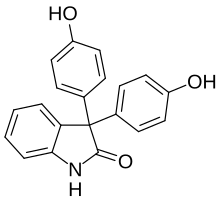
Oxyphenisatine
Long-term use is associated with liver damage,[4] and as a result, it was withdrawn in most countries in the early 1970s.
Natural chemical compounds similar to oxyphenisatine may be present in prunes,[5] but a recent review of the relevant scientific literature suggests that the laxative effect of prunes is due to other constituents including phenolic compounds (mainly neochlorogenic acids and chlorogenic acids) and sorbitol.
The ketone group of isatin (1) is nonenolizable and has interesting properties.
In strong acid it becomes protonated, and the oxygen can be replaced by electron rich moieties.
In 1885, it was reported that condensation of isatin with phenol 2 leads to oxyphenisatin (3), which can then also be acetylated to (4).