Ozone
In 1873 James Dewar and John Gray McKendrick documented that frogs grew sluggish, birds gasped for breath, and rabbits' blood showed decreased levels of oxygen after exposure to "ozonized air", which "exercised a destructive action".
[22][12] Schönbein himself reported that chest pains, irritation of the mucous membranes, and difficulty breathing occurred as a result of inhaling ozone, and small mammals died.
The only thoroughly well-ascertained knowledge concerning the physiological effect of ozone, so far attained, is that it causes irritation and œdema of the lungs, and death if inhaled in relatively strong concentration for any time.
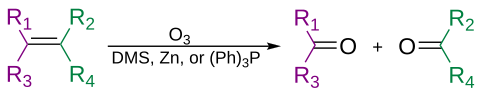
[12][25] Until the 1920s, it was not certain whether small amounts of oxozone, O4, were also present in ozone samples due to the difficulty of applying analytical chemistry techniques to the explosive concentrated chemical.
[26][27] In 1923, Georg-Maria Schwab (working for his doctoral thesis under Ernst Hermann Riesenfeld) was the first to successfully solidify ozone and perform accurate analysis which conclusively refuted the oxozone hypothesis.
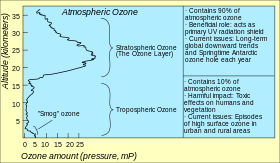
In the second half of the 20th century, the amount of ozone in the stratosphere was discovered to be declining, mostly because of increasing concentrations of chlorofluorocarbons (CFC) and similar chlorinated and brominated organic molecules.
The concern over the health effects of the decline led to the 1987 Montreal Protocol, the ban on the production of many ozone-depleting chemicals and in the first and second decade of the 21st century the beginning of the recovery of stratospheric ozone concentrations.
The small unabsorbed part that remains of UV-B after passage through ozone causes sunburn in humans, and direct DNA damage in living tissues in both plants and animals.
Ozone photolysis by UV light leads to production of the hydroxyl radical HO• and this plays a part in the removal of hydrocarbons from the air, but is also the first step in the creation of components of smog such as peroxyacyl nitrates, which can be powerful eye irritants.
[57] There is evidence of significant reduction in agricultural yields because of increased ground-level ozone and pollution which interferes with photosynthesis and stunts overall growth of some plant species.
[67] According to an article from the University of Colorado-Boulder, "Oil and natural gas VOC emission have a major role in ozone production and bear the potential to contribute to elevated O3 levels in the Northern Colorado Front Range (NCFR)".
[86] Neutrophils, another important cell type of the innate immune system that primarily targets bacterial pathogens,[88] are found to be present in the airways within 6 hours of exposure to high ozone levels.
[87][88] Lymphocytes, a cellular component of the adaptive immune response, produce an increased amount of inflammatory chemicals called "cytokines" after exposure to ozone, which may contribute to airway hyperreactivity and worsening asthma symptoms.
In normal tissue, the epithelial layer forms a protective barrier, and also contains specialized ciliary structures that work to clear foreign bodies, mucus and pathogens from the lungs.
[43] Furthermore, another report states that "results of some controlled studies show that concentrations of ozone considerably higher than these [human safety] standards are possible even when a user follows the manufacturer's operating instructions".
The study revealed that people living in cities with high ozone levels, such as Houston or Los Angeles, had an over 30% increased risk of dying from lung disease.
[114] On January 7, 2010, the U.S. Environmental Protection Agency (EPA) announced proposed revisions to the National Ambient Air Quality Standard (NAAQS) for the pollutant ozone, the principal component of smog: ... EPA proposes that the level of the 8-hour primary standard, which was set at 0.075 μmol/mol in the 2008 final rule, should instead be set at a lower level within the range of 0.060 to 0.070 μmol/mol, to provide increased protection for children and other at risk populations against an array of O3 – related adverse health effects that range from decreased lung function and increased respiratory symptoms to serious indicators of respiratory morbidity including emergency department visits and hospital admissions for respiratory causes, and possibly cardiovascular-related morbidity as well as total non- accidental and cardiopulmonary mortality ...[115]On October 26, 2015, the EPA published a final rule with an effective date of December 28, 2015, that revised the 8-hour primary NAAQS from 0.075 ppm to 0.070 ppm.
[125][126] A 2022 study concludes that East Asia loses 63 billion dollars in crops per year due to ozone pollution, a byproduct of fossil fuel combustion.
The Canadian Centre for Occupation Safety and Health reports that: Even very low concentrations of ozone can be harmful to the upper respiratory tract and the lungs.
"[129]To protect workers potentially exposed to ozone, U.S. Occupational Safety and Health Administration has established a permissible exposure limit (PEL) of 0.1 μmol/mol (29 CFR 1910.1000 table Z-1), calculated as an 8-hour time weighted average.
Use of an oxygen concentrator can further increase the ozone production and further reduce the risk of nitric acid formation by removing not only the water vapor, but also the bulk of the nitrogen.
Several megawatts of electrical power may be installed in large facilities, applied as single phase AC current at 50 to 8000 Hz and peak voltages between 3,000 and 20,000 volts.
Because of the high reactivity of ozone, only a few materials may be used like stainless steel (quality 316L), titanium, aluminium (as long as no moisture is present), glass, polytetrafluorethylene, or polyvinylidene fluoride.
[142] In the laboratory, ozone can be produced by electrolysis using a 9 volt battery, a pencil graphite rod cathode, a platinum wire anode, and a 3 molar sulfuric acid electrolyte.
In its simplest form, high voltage AC, such as the output of a neon-sign transformer is connected to two metal rods with the ends placed sufficiently close to each other to allow an arc.
Where electrical power is abundant, ozone is a cost-effective method of treating water, since it is produced on demand and does not require transportation and storage of hazardous chemicals.
[159][160] Despite this, with research revealing the negative impacts of common disinfectants like chlorine with respect to toxic residuals and ineffectiveness in killing certain micro-organisms,[161] DBD plasma-based ozone decontamination is of interest in current available technologies.
[160][162][163][164] Advantages of ozone include high thermodynamic oxidation potential, less sensitivity to organic material and better tolerance for pH variations while retaining the ability to kill bacteria, fungi, viruses, as well as spores and cysts.
Studies at California Polytechnic University demonstrated that 0.3 μmol/mol levels of ozone dissolved in filtered tapwater can produce a reduction of more than 99.99% in such food-borne microorganisms as salmonella, E. coli 0157:H7 and Campylobacter.
[177][178] Ozone is used in homes and hot tubs to kill bacteria in the water and to reduce the amount of chlorine or bromine required by reactivating them to their free state.