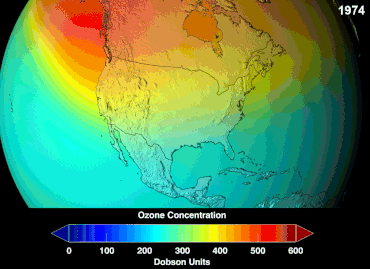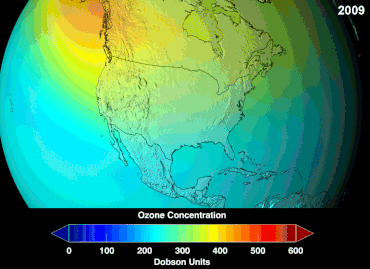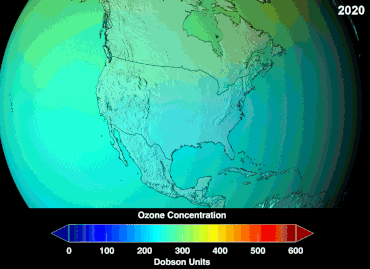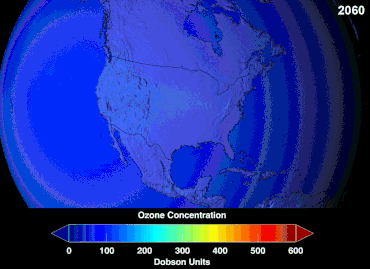
Ozone depletion
These wavelengths cause skin cancer, sunburn, permanent blindness, and cataracts,[5] which were projected to increase dramatically as a result of thinning ozone, as well as harming plants and animals.
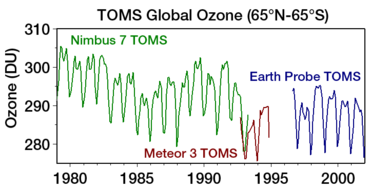
Ozone levels stabilized by the mid-1990s and began to recover in the 2000s, as the shifting of the jet stream in the southern hemisphere towards the south pole has stopped and might even be reversing.
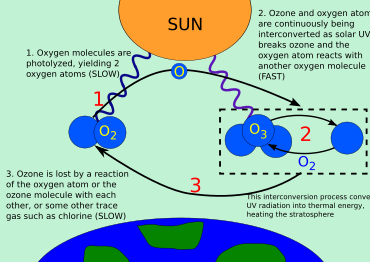
Once in the stratosphere, the Cl and Br atoms are released from the parent compounds by the action of ultraviolet light, e.g. Ozone is a highly reactive molecule that easily reduces to the more stable oxygen form with the assistance of a catalyst.
Early models failed to take PSCs into account and predicted a gradual global depletion, which is why the sudden Antarctic ozone hole was such a surprise to many scientists.
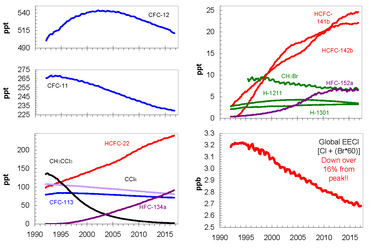
[40] A study by an international team of researchers published in Nature found that since 2013 emissions that are predominately from north-eastern China have released large quantities of the banned chemical Chlorofluorocarbon-11 (CFC-11) into the atmosphere.
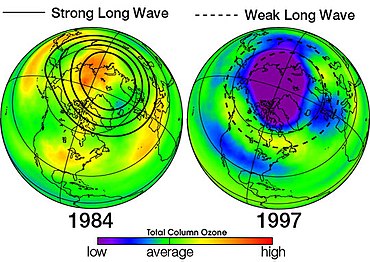
[47] The ozone hole occurs during the Antarctic spring, from September to early December, as strong westerly winds start to circulate around the continent and create an atmospheric container.
[51] During the Antarctic winter and spring, reactions on the surface of the polar stratospheric cloud particles convert these "reservoir" compounds into reactive free radicals (Cl and ClO).
As the ozone hole over Antarctica has in some instances grown so large as to affect parts of Australia, New Zealand, Chile, Argentina, and South Africa, environmentalists have been concerned that the increase in surface UV could be significant.
[59] Excessive ultraviolet radiation (UVR) has reducing effects on the rates of photosynthesis and growth of benthic diatom communities (microalgae species that increase water quality and are pollution resistant) that are present in shallow freshwater.
By combining epidemiological data with results of animal studies, scientists have estimated that every one percent decrease in long-term stratospheric ozone would increase the incidence of these cancers by 2%.
[90] At the time this was widely regarded as a first step towards a more comprehensive regulation policy, but progress in this direction slowed in subsequent years, due to a combination of political factors (continued resistance from the halocarbon industry and a general change in attitude towards environmental regulation during the first two years of the Reagan administration) and scientific developments (subsequent National Academy assessments that indicated that the first estimates of the magnitude of ozone depletion had been overly large).
[91] The U.S. government's attitude began to change again in 1983, when William Ruckelshaus replaced Anne M. Burford as Administrator of the United States Environmental Protection Agency (EPA).
In 1985 twenty nations, including most of the major CFC producers, signed the Vienna Convention for the Protection of the Ozone Layer, which established a framework for negotiating international regulations on ozone-depleting substances.
[90] After a series of scientific expeditions to the Antarctic produced convincing evidence that the ozone hole was indeed caused by chlorine and bromine from manmade organohalogens, the Montreal Protocol was strengthened at a 1990 meeting in London.
The participants agreed to phase out CFCs and halons entirely (aside from a very small amount marked for certain "essential" uses, such as asthma inhalers) by 2000 in non-Article 5 countries and by 2010 in Article 5 (less developed) signatories.
[95][96] Civil society, including especially non-governmental organizations (NGOs), played critical roles at all stages of policy development leading to the Vienna Conference, the Montreal Protocol, and in assessing compliance afterwards.
The reduction of the radiative forcing due to ODS probably masked the true level of climate change effects of other greenhouse gases, and was responsible for the "slow down" of global warming from the mid-90s.
In the 1950s, David Bates and Marcel Nicolet presented evidence that various free radicals, in particular hydroxyl (OH) and nitric oxide (NO), could catalyze this recombination reaction, reducing the overall amount of ozone.
In the following year, Crutzen and (independently) Harold Johnston suggested that NO emissions from supersonic passenger aircraft, which would fly in the lower stratosphere, could also deplete the ozone layer.
[126] In 1974 Frank Sherwood Rowland, Chemistry Professor at the University of California at Irvine, and his postdoctoral associate Mario J. Molina suggested that long-lived organic halogen compounds, such as CFCs, might behave in a similar fashion as Crutzen had proposed for nitrous oxide.
[134] Alternative hypotheses, which had attributed the ozone hole to variations in solar UV radiation or to changes in atmospheric circulation patterns, were also tested and shown to be untenable.
Since 1981 the United Nations Environment Programme, under the auspices of the World Meteorological Organization, has sponsored a series of technical reports on the Scientific Assessment of Ozone Depletion, based on satellite measurements.
"[137] In 2012, NOAA and NASA reported "Warmer air temperatures high above the Antarctic led to the second smallest season ozone hole in 20 years averaging 17.9 million square kilometres.
"[148] The study analyzed data from the Aura and CALIPSO satellites, and determined that the larger-than-normal ozone loss was due to an unusually long period of cold weather in the Arctic, some 30 days more than typical, which allowed for more ozone-destroying chlorine compounds to be created.
Specifically, Lu's work defines "ozone hole" as "an area with O3 loss in percent larger than 25%, with respect to the undisturbed O3 value when there were no significant CFCs in the stratosphere (~ in the 1960s)"[163] instead of the general definition of 220 Dobson units or lower.
Analyzing the atmospheric impacts of the 2019–2020 Australian bushfire season, scientists led by MIT researcher Susan Solomon found the smoke destroyed 3–5% of ozone in affected areas of the Southern Hemisphere.
[87] Prior to the 1980s, the EU, NASA, NAS, UNEP, WMO and the British government had dissenting scientific reports and Watson played a role in the process of unified assessments.
A similar erroneous assertion is that soluble halogen compounds from the volcanic plume of Mount Erebus on Ross Island, Antarctica are a major contributor to the Antarctic ozone hole.
Based on the NCEP/NCAR reanalysis data over the last 35 years and by using the NOAA HYSPLIT trajectory model, researchers showed that Erebus volcano gas emissions (including hydrogen chloride (HCl)) can reach the Antarctic stratosphere via high-latitude cyclones and then the polar vortex.
[88] The ozone hole was seen as a "hot issue" and imminent risk[184] as laypeople feared severe personal consequences such as skin cancer, cataracts, damage to plants, and reduction of plankton populations in the ocean's photic zone.