Ozone layer
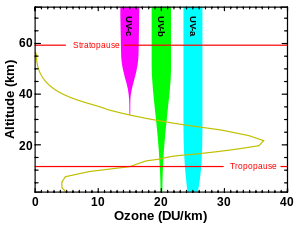
The ozone layer is mainly found in the lower portion of the stratosphere, from approximately 15 to 35 kilometers (9 to 22 mi) above Earth, although its thickness varies seasonally and geographically.
[3] Its properties were explored in detail by the British meteorologist G. M. B. Dobson, who developed a simple spectrophotometer (the Dobsonmeter) that could be used to measure stratospheric ozone from the ground.
[4] In 1985, atmospheric research revealed that the ozone layer was being depleted by chemicals released by industry, mainly chlorofluorocarbons (CFCs).
The United Nations General Assembly has designated September 16 as the International Day for the Preservation of the Ozone Layer.
[11] Research has found that the ozone levels in the United States are highest in the spring months of April and May and lowest in October.
While there are natural sources for all of these species, the concentrations of chlorine and bromine increased markedly in recent decades because of the release of large quantities of man-made organohalogen compounds, especially chlorofluorocarbons (CFCs) and bromofluorocarbons.
[14] Atmospheric components are not sorted out by weight in the homosphere because of wind-driven mixing that extends to an altitude of about 90 km, well above the ozone layer.
So despite being heavier than diatomic nitrogen and oxygen, these highly stable compounds rise into the stratosphere, where Cl and Br radicals are liberated by the action of ultraviolet light.
For approximately 5 percent of the Earth's surface, around the north and south poles, much larger seasonal declines have been seen, and are described as "ozone holes".
[16] The discovery of the annual depletion of ozone above the Antarctic was first announced by Joe Farman, Brian Gardiner, and Jonathan Shanklin, in a paper which appeared in Nature on May 16, 1985.
Regulation attempts have included but not have been limited to the Clean Air Act implemented by the United States Environmental Protection Agency.
[17] Effective presentation of information has also proven to be important in order to educate the general population of the existence and regulation of ozone depletion and contaminants.
A scientific paper was written by Sheldon Ungar in which the author explores and studies how information about the depletion of the ozone, climate change, and various related topics.
The ozone case was communicated to lay persons "with easy-to-understand bridging metaphors derived from the popular culture" and related to "immediate risks with everyday relevance".
[22] In 1978, the United States, Canada, and Norway enacted bans on CFC-containing aerosol sprays that damage the ozone layer but the European Community rejected a similar proposal.
After negotiation of an international treaty (the Montreal Protocol), CFC production was capped at 1986 levels with commitments to long-term reductions.
[26] On August 2, 2003, scientists announced that the global depletion of the ozone layer might be slowing because of the international regulation of ozone-depleting substances.
In a study organized by the American Geophysical Union, three satellites and three ground stations confirmed that the upper-atmosphere ozone-depletion rate slowed significantly over the previous decade.
The Galaxy Evolution Explorer, GALEX, is an orbiting ultraviolet space telescope launched on April 28, 2003, which operated until early 2012.