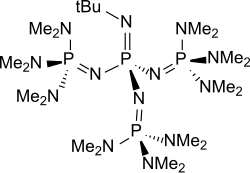
P4-t-Bu
P4-t-Bu is a readily accessible chemical from the group of neutral, peralkylated sterically hindered polyaminophosphazenes, which are extremely strong bases but very weak nucleophiles, with the formula (CH3)3C−N=P(−N=P(−N(CH3)2)3)3.
[9] The transfer of the hygroscopic and readily water-soluble hydrochlorides and the liquid free bases into the tetrafluoroborates, which are difficult to solubilize in water, facilitate the handling of the substances considerably.
The relatively uncomplicated convergent synthesis with easily accessible reactants and very good yields of the intermediates make P4-t-Bu an interesting phosphazene superbase.
The base is both very hydrophilic and very lipophilic and can be recovered easily and almost completely from reaction mixtures by the formation of the sparingly soluble tetrafluoroborate salt.
[12] Alkylations on weakly acidic methylene groups (e.g. in the case of carboxylic esters or nitriles) proceed with high yield and selectivity.
[13] Succinonitrile reacts with iodoethane in the presence of P4-t-Bu in 98% yield to give the tetraethyl derivative without undergoing a Thorpe-Ziegler reaction to form a cyclic α-ketonitrile.
[9] Trifluoromethylation of ketones (such as benzophenone) is also possible at room temperature in good yields up to 84% with the inert fluoroform (HFC-23) in the presence of P4-t-Bu and tris(trimethylsilyl)amine.
With the ethyl acetate/P4-t-Bu initiator system, poly(methyl methacrylate) (PMMA) with narrow polydispersity and molar masses up to 40,000 g·mol−1 could be obtained in THF.
[16] Cyclic siloxanes (such as hexamethylcyclotrisiloxane or decamethylcyclopentasiloxane) can also be polymerized with catalytic amounts of P4-t-Bu and water or silanols as initiators under good molecular weight control to thermally very stable polysiloxanes having decomposition temperatures of >450 °C.
The extreme hygroscopy of the phosphazene base P4-t-Bu as a substance and in solutions requires a great effort for storage and handling and prevents its broader use.