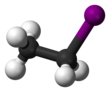
Ethyl iodide
Ethyl iodide (also iodoethane) is a colorless flammable chemical compound.
It has the chemical formula C2H5I and is prepared by heating ethanol with iodine and phosphorus.
[2] On contact with air, especially on the effect of light, it decomposes and turns yellow or reddish from dissolved iodine.
Ethyl iodide should be stored in the presence of copper powder to avoid rapid decomposition, though even with this method samples do not last more than 1 year.
Ethyl iodide is prepared by using red phosphorus, absolute ethanol and iodine.