Polymerase chain reaction
Mullis and biochemist Michael Smith, who had developed other essential ways of manipulating DNA, were jointly awarded the Nobel Prize in Chemistry in 1993.
[1] PCR is fundamental to many of the procedures used in genetic testing and research, including analysis of ancient samples of DNA and identification of infectious agents.
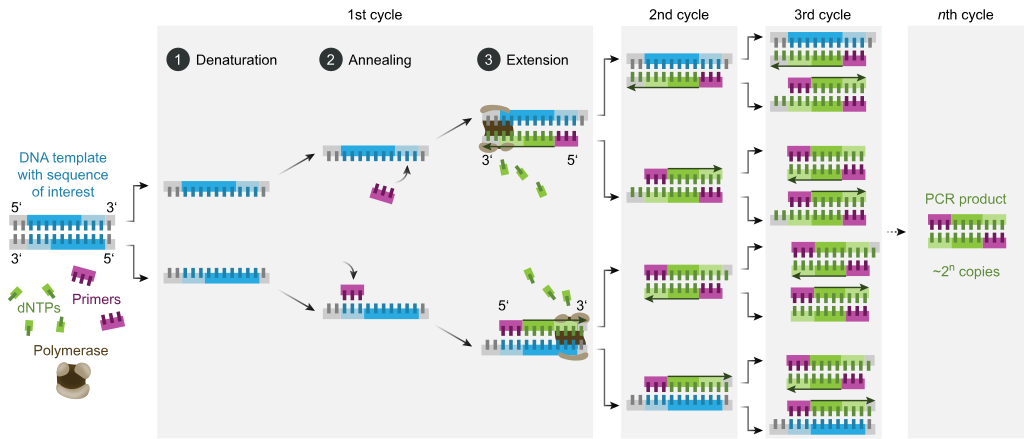
In the first step of PCR, the two strands of the DNA double helix are physically separated at a high temperature in a process called nucleic acid denaturation.
Older thermal cyclers lacking a heated lid require a layer of oil on top of the reaction mixture or a ball of wax inside the tube.
Hence the PCR-setup areas is separated from the analysis or purification of other PCR products, disposable plasticware used, and the work surface between reaction setups needs to be thoroughly cleaned.
[19][20] Both the running parameters (e.g. temperature and duration of cycles), or the addition of reagents, such as formamide, may increase the specificity and yield of PCR.
[23] PCR may also be used for genetic fingerprinting; a forensic technique used to identify a person or organism by comparing experimental DNAs through different PCR-based methods.
[citation needed] Some PCR fingerprint methods have high discriminative power and can be used to identify genetic relationships between individuals, such as parent-child or between siblings, and are used in paternity testing (Fig.
These PCR-based techniques have been successfully used on animals, such as a forty-thousand-year-old mammoth, and also on human DNA, in applications ranging from the analysis of Egyptian mummies to the identification of a Russian tsar and the body of English king Richard III.
qPCR allows the quantification and detection of a specific DNA sequence in real time since it measures concentration while the synthesis process is taking place.
This technique lowers the possibility of error at the end point of PCR,[27] increasing chances for detection of genes associated with genetic diseases such as cancer.
The mathematical foundations for the reliable quantification of the PCR[28] and RT-qPCR[29] facilitate the implementation of accurate fitting procedures of experimental data in research, medical, diagnostic and infectious disease applications.
[36] PCR also permits identification of non-cultivatable or slow-growing microorganisms such as mycobacteria, anaerobic bacteria, or viruses from tissue culture assays and animal models.
The basis for PCR diagnostic applications in microbiology is the detection of infectious agents and the discrimination of non-pathogenic from pathogenic strains by virtue of specific genes.
The technique is highly sensitive with the potential to produce millions to billions of copies of a specific product for sequencing, cloning, and analysis.
If the procedure can be further simplified and sensitive non-radiometric detection systems can be developed, the PCR will assume a prominent place in the clinical laboratory for years to come.
[16] One major limitation of PCR is that prior information about the target sequence is necessary in order to generate the primers that will allow its selective amplification.
[citation needed] Like all enzymes, DNA polymerases are also prone to error, which in turn causes mutations in the PCR fragments that are generated.
[citation needed] The heat-resistant enzymes that are a key component in polymerase chain reaction were discovered in the 1960s as a product of a microbial life form that lived in the superheated waters of Yellowstone's Mushroom Spring.
[81] A 1971 paper in the Journal of Molecular Biology by Kjell Kleppe and co-workers in the laboratory of H. Gobind Khorana first described a method of using an enzymatic assay to replicate a short DNA template with primers in vitro.
[82] However, this early manifestation of the basic PCR principle did not receive much attention at the time and the invention of the polymerase chain reaction in 1983 is generally credited to Kary Mullis.
[83][page needed] When Mullis developed the PCR in 1983, he was working in Emeryville, California for Cetus Corporation, one of the first biotechnology companies, where he was responsible for synthesizing short chains of DNA.
[88] Mullis's 1985 paper with R. K. Saiki and H. A. Erlich, "Enzymatic Amplification of β-globin Genomic Sequences and Restriction Site Analysis for Diagnosis of Sickle Cell Anemia"—the polymerase chain reaction invention (PCR)—was honored by a Citation for Chemical Breakthrough Award from the Division of History of Chemistry of the American Chemical Society in 2017.
The DNA polymerases initially employed for in vitro experiments presaging PCR were unable to withstand these high temperatures.







- Father
- Child
- Mother
The child has inherited some, but not all, of the fingerprints of each of its parents, giving it a new, unique fingerprint.


