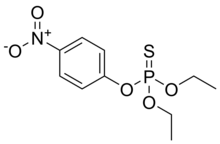
Parathion
After an insect (or a human) ingests parathion, an oxidase replaces the double bonded sulfur with oxygen to give paraoxon.
Hydrolysis, which deactivates the molecule, occurs at the aryl ester bond resulting in diethyl thiophosphate and 4-nitrophenol.
[8] Degradation proceeds differently under anaerobic conditions: the nitro group on parathion is reduced to the amine.
Paraoxon exposure can result in headaches, convulsions, poor vision, vomiting, abdominal pain, severe diarrhea, unconsciousness, tremor, dyspnea, and finally pulmonary edema as well as respiratory arrest.
If human poisoning is detected early and the treatment is prompt (atropine and artificial respiration), fatalities are infrequent.
Peripheral neuropathy including paralysis is noticed as late sequelae after recovery from acute intoxication.
Parathion was used as a chemical warfare agent, most notably by an element of the British South Africa Police attached to the Selous Scouts during the Rhodesian Bush War.
[10][11][12] Based on animal studies, parathion is considered by the U.S. Environmental Protection Agency to be a possible human carcinogen.
Industrial safety during the production process requires special ventilation and continuous measurement of air contamination in order not to exceed PEL levels, as well as careful attention to personal hygiene.
Frequent analysis of workers' serum acetylcholinesterase activity is also helpful with regards to occupational safety, because the action of parathion is cumulative.
to find the most lethal means of exposure to humans, intending to take an antidote afterwards, but was paralyzed and so died before he could reach it.