Particle aggregation
Particle agglomeration refers to the formation of assemblages in a suspension and represents a mechanism leading to the functional destabilization of colloidal systems.
Particle agglomeration can be induced by adding salts or other chemicals referred to as coagulant or flocculant.
Alternatively, a colloidal gel may form in concentrated suspensions which changes its rheological properties.
Colloidal particles may also remain dispersed in liquids for long periods of time (days to years).
This parameter provides a readily quantifiable measure of interparticle repulsion, which is the key inhibitor of particle aggregation.
A well dispersed colloidal suspension consists of individual, separated particles and is stabilized by repulsive inter-particle forces.
Since absolute aggregation rates are difficult to measure, one often refers to the dimensionless stability ratio W, defined as
When salt is added to the suspension, the electrical double layer repulsion is screened, and van der Waals attraction become dominant and induce fast aggregation.
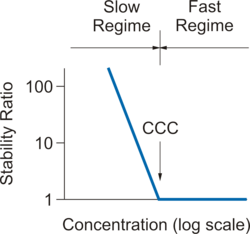
The figure on the right shows the typical dependence of the stability ratio W versus the electrolyte concentration, whereby the regimes of slow and fast aggregation are indicated.
The table below summarizes the critical coagulation concentration (CCC) ranges for different net charge of the counter ion.
This dependence reflects the Schulze–Hardy rule,[5][6] which states that the CCC varies as the inverse sixth power of the counter ion charge.
Since particles are frequently negatively charged, multivalent metal cations thus represent highly effective coagulants.
Adsorbed or grafted polymers may form a protective layer around the particles, induce steric repulsive forces, and lead to steric stabilization at it is the case with polycarboxylate ether (PCE), the last generation of chemically tailored superplasticizer specifically designed to increase the workability of concrete while reducing its water content to improve its properties and durability.
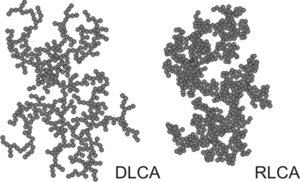
When aggregation occurs in a suspension composed of similar monodisperse colloidal particles, the process is called homoaggregation (or homocoagulation).
When aggregation occurs in a suspension composed of dissimilar colloidal particles, one refers to heteroaggregation (or heterocoagulation).
The variation of transmitted light through an aggregating suspension can be studied with a regular spectrophotometer in the visible region.
The increase of the absorbance can be related to the aggregation rate constant k and the stability ratio can be estimated from such measurements.
Aggregation processes in strongly scattering systems have been studied with transmittance, backscattering techniques or diffusing-wave spectroscopy.
This technique offers excellent resolution, whereby clusters made out of tenths of particles can be resolved individually.
While it can be difficult to obtain quantitative information on aggregation rates or cluster properties from such experiments, they can be most valuable for practical applications.
Automated instruments based on light scattering/transmittance to monitor suspension settling have been developed, and they can be used to probe particle aggregation.
Other indirect techniques capable to monitor the state of aggregation include, for example, filtration, rheology, absorption of ultrasonic waves, or dielectric properties.
[10] Particle aggregation is a widespread phenomenon, which spontaneously occurs in nature but is also widely explored in manufacturing.
Treatment of municipal waste water normally includes a phase where fine solid particles are removed.
This separation is achieved by addition of a flocculating or coagulating agent, which induce the aggregation of the suspended solids.
Commonly used flocculating agents in water treatment include multivalent metal ions (e.g., Fe3+ or Al3+), polyelectrolytes, or both.
The key step in cheese production is the separation of the milk into solid curds and liquid whey.
This separation is achieved by inducing the aggregation processes between casein micelles by acidifying the milk or adding rennet.