Payne rearrangement
Although the migration itself is fully reversible, nucleophilic opening under Curtin–Hammett conditions provides good yields of functionalized diols derived from a single epoxy alcohol isomer.
(1)Strongly basic conditions are required to induce equilibration, which limits the synthetic utility of the transformation to substrates lacking base-labile functionality.
Many epoxy alcohol equilibria are very finely balanced;[3] however, taking advantage of the trapping strategies described above may lead to high yields of single isomers.
The basic mechanism of the Payne rearrangement involves deprotonation of the free hydroxyl group, invertive nucleophilic attack on the proximal epoxide carbon, and re-protonation of the newly freed alkoxide.
Substrates containing multiple adjacent hydroxyl groups may undergo "cascade" epoxide migrations with inversion at each site of nucleophilic attack.
Nucleophiles that may be used under protic conditions include phenols, secondary amines, azide anion, and sulfides.
[11] (8)Intermolecular nucleophilic trapping of a single epoxide isomer is difficult, as reaction of the epoxy alcohol with the electrophile is typically faster than migration.
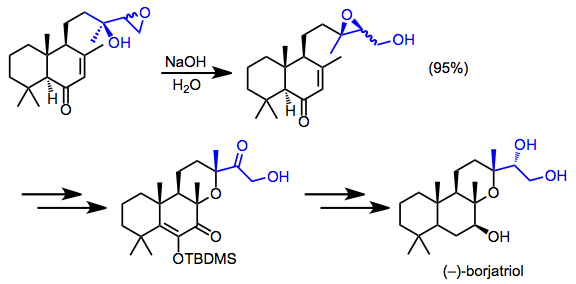
[16] (12)The final two steps in the total synthesis of spatol involved intramolecular electrophilic trapping of an alkoxide derived from a rearranged epoxide.
Attack of the intermediate alkoxide on the adjacent mesylate afforded a bis(epoxide), and debenzylation provided the target compound.
[11] (15)Opening of terminal epoxides by adventitious hydroxide may occur under the conditions of rearrangement; if this is not desired, anhydrous solvents, reagents, and glassware must be used.
Nucleophilic opening can be accomplished through the use of sodium azide, excess hydroxide, or cuprate reagents in the presence of lithium chloride.
The reaction mixture was then stirred for a further 12 hours and then cautiously treated with 5 mL of saturated aqueous ammonium chloride.