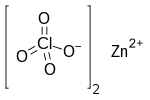
Zinc perchlorate
Zinc perchlorate is the inorganic compound with the chemical formula Zn(ClO4)2 which forms the hexahydrate.
[1][2] Zinc perchlorate can be prepared by dissolving zinc oxide or zinc carbonate in perchloric acid:[3] The compound decomposes when heated to high temperatures and may explode if heated too strongly.
Zinc perchlorate can form complexes with ligands such as 8-aminoquinoline, tricarbohydrazide, and tetraphenylethylene tetratriazole.
[5][6] Zinc perchlorate forms a hygroscopic colorless solid, odorless, soluble in water and low-weight alcohols.
Zinc perchlorate is used as an oxidizing agent and catalyst.