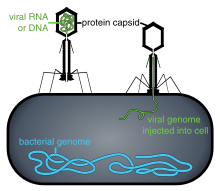
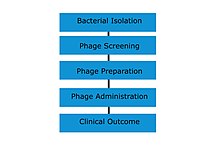
Phage therapy
[30] Isolated from Western advances in antibiotic production in the 1940s, Soviet scientists continued to develop already successful phage therapy to treat the wounds of soldiers in field hospitals.
[25][24] As a result of the development of antibiotic resistance since the 1950s and an advancement of scientific knowledge, there has been renewed interest worldwide in the ability of phage therapy to eradicate bacterial infections and chronic polymicrobial biofilm (including in industrial situations).
[37] Some of the interest in the West can be traced back to 1994, when James Soothill demonstrated (in an animal model) that the use of phages could improve the success of skin grafts by reducing the underlying Pseudomonas aeruginosa infection.
[47] In January 2016, phages were used successfully at Yale University by Benjamin Chan to treat a chronic Pseudomonas aeruginosa infection in ophthalmologist Ali Asghar Khodadoust.
[citation needed] In 2017, a pair of genetically engineered phages along with one naturally occurring (so-called "phage Muddy") each from among those catalogued by SEA-PHAGES (Science Education Alliance-Phage Hunters Advancing Genomics and Evolutionary Science) at the Howard Hughes Medical Institute by Graham Hatfull and colleagues, was used by microbiologist James Soothill at Great Ormond Street Hospital for Children in London to treat an antibiotic-resistant bacterial (Mycobacterium abscessus) infection in a young woman with cystic fibrosis.
[49][50][51][52] In 2022, two mycobacteriophages were administered intravenously twice daily to a young man with treatment-refractory Mycobacterium abscessus pulmonary infection and severe cystic fibrosis lung disease.
[53] In a second case, successful treatment of disseminated cutaneous Mycobacterium chelonae was reported with a single phage administered intravenously twice daily in conjunction with antibiotic and surgical management.
[68] Funding for phage therapy research and clinical trials is generally insufficient and difficult to obtain, since it is a lengthy and complex process to patent bacteriophage products.
[70][71][72][73] Phase-1 clinical trials were conducted at the Southwest Regional Wound Care Center of Lubbock, Texas, for a cocktail of phages against P. aeruginosa, Staphylococcus aureus, and Escherichia coli, developed by Intralytix.
[medical citation needed] In February 2020, the FDA approved a clinical trial to evaluate bacteriophage therapy in patients with urinary tract infections.
[48] Intravenous phage drip therapy was successfully used to treat a patient with multidrug-resistant Acinetobacter baumannii in Thornton Hospital at UC San Diego in 2017.
[92] Nebulized phage therapy has been used successfully to treat numerous patients with cystic fibrosis and multidrug-resistant bacteria at Yale University as part of their compassionate use program.
[93][94] In 2019, a Brownsville, Minnesota resident with a longstanding bacterial infection in his knee received a phage treatment at the Mayo Clinic that eliminated the need for amputation of his lower leg.
Additionally, patent issues (specifically on living organisms) may complicate distribution for pharmaceutical companies wishing to have exclusive rights over their "invention", which would discourage a commercial corporation from investing capital in this.
[108][111] Moreover, it has been shown that the evolution of bacterial resistance to phage attack changes the efflux pump mechanism, causing increased sensitivity to drugs from several antibiotic classes.
However, we should also consider the fact that many phage resistance systems are mounted on mobile genetic elements, including prophages and plasmids, and thus may spread quite rapidly even without direct selection.
[medical citation needed] Due to many experimental treatments in human patients conducted in past decades, and to already existing RCTs (see section: Clinical experience and randomized controlled trials), phage safety can be assessed directly.
[121][137] Intravenous administration of bacteriophages is conducted under strict medical supervision, by specialists in infectious diseases within a hospital setting, due to potential adverse reactions.
[142] Continuous monitoring of heart rate, blood pressure, and temperature to detect early signs of adverse reactions is done after the intravenous phage administration.
Even lambda, a temperate phage of the E. coli K-12 laboratory strain, carries two genes that provide potential virulence benefits to the lysogenic host, one that increases intestinal adherence and the other that confers resistance to complement killing in the blood.
[155] Janakiraman Ramachandran[32] argues that this complication can be avoided in those types of infection where this reaction is likely to occur by using genetically engineered bacteriophages that have had their gene responsible for producing endolysin removed.
[17] Eventually, these dead cells are consumed by the normal house-cleaning duties of the phagocytes, which utilize enzymes to break down the whole bacterium and its contents into harmless proteins, polysaccharides, and lipids.
Phage therapy performed at the PTU is considered an "experimental treatment", covered by the adapted Act of 5 December 1996 on the Medical Profession (Polish Law Gazette, 2011, No.
Much like Article 37 of the Helsinki Declaration, the compassionate use treatment option can only be applied when the phages are expected to help in life-threatening or chronic and/or seriously debilitating diseases that are not treatable with formally approved products.
[citation needed] In France, ANSM, the French medicine agency, has organized a specific committee—Comité Scientifique Spécialisé Temporaire (CSST)—for phage therapy, which consists of experts in various fields.
[168] In Belgium, in 2016 and in response to a number of parliamentary questions, Maggie De Block, the Minister of Social Affairs and Health, acknowledged that it is indeed not evident to treat phages as industrially made drugs, and therefore she proposed to investigate if the magistral preparation pathway could offer a solution.
Phage active pharmaceutical ingredients to be included in magistral preparations must meet the requirements of a monograph, which describes their production and quality control testing.
[170] However, many regulations were not yet established back then, and phage therapy soon lost people's interest due to the prevalence of antibiotics, which eventually led to the antimicrobial resistance crisis.
It describes Strathdee's ultimately successful attempt to introduce phage therapy as a life-saving treatment for her husband, critically ill with a completely antibiotic-resistant Acinetobacter baumannii infection following severe pancreatitis.
This article was adapted from the following source under a CC BY 4.0 license (2021) (reviewer reports): Joana Azeredo, Jean-Paul Pirnay, Diana Priscila Pires, Mzia Kutateladze, Krystyna Dabrowska, Rob Lavigne, Bob G Blasdel (15 December 2021).