Phantom pain
[2] It is typically a manifestation of an underlying source, such as surgical trauma, neuroma formation, infection, or an improperly fitted prosthetic device.
[10] Sensations may be described as shooting, stabbing, squeezing, throbbing, tingling, or burning, and sometimes feels as if the phantom part is being forced into an uncomfortable position.
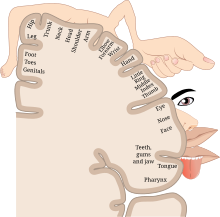
[12] It is thought that phantom pain more commonly involves the part of the limb farthest from the body because of its larger cortical representation within the somatosensory cortex.
[2] Phantom pain is seen more often in older adults as compared to individuals with congenital limb deficiency or amputation at an early age.
[17] Currently, theories are based on altered neurological pathways and maladaptive changes within the peripheral nervous system, spinal cord, and brain.
Neuromas formed from injured nerve endings at the stump site show increased sodium channel expression and are able to spontaneously fire abnormal action potentials.
However, it has been noted that pain still persists once the neuromas have ceased firing action potentials or when peripheral nerves are treated with conduction blocking agents.
These changes to the nerve fiber inputs may also lead to an expansion of the neuronal receptive fields, such that previously non-noxious stimuli are now interpreted as noxious.
[1] It is also known that increased expression of glutamate and NMDA, coupled with decreased inhibition from GABAergic neurons, further contributes to the mechanism of central sensitization.
[19] However, because patients with complete spinal cord injury have experienced phantom pain, there must also be an underlying central mechanism within the brain.
For much of the twentieth century, it was believed that no new neural circuits could be formed in the adult mammalian brain, but experiments from the 1980s onward cast this into doubt.
[21] This leads to areas of the brain formerly receiving input from the lost limb now able to be stimulated from the nearby invading cortical regions.
[17] Additionally, as phantom pains in upper extremity amputees increased, there was a higher degree of medial shift of the facial motor representation.
Evaluation of the residual limb should be done to inspect for signs of infection, bursa or pressure ulcer formation, or deep tissue injury.
A thorough neurological and musculoskeletal examination should be conducted, including assessment of strength, range of motion, and reflexes, to rule out any other central or peripheral causes for the pain.
[27] Doctors may prescribe medications, and some antidepressants or antiepileptics have been shown to have a beneficial effect on reducing phantom limb pain.
[33][34] N-methyl-D-aspartate (NMDA) receptor antagonists, such as ketamine, are thought to work by reversing the process of central sensitization within the spinal cord, which has been proposed as a possible mechanism for the development of phantom pain.
[11] The use of ketamine has shown to reduce phantom pain, but memantine, a medication within the same drug class, did not provide any benefit to patients.
According to a 2017 paper that reviewed a wide range of studies using mirror therapy, patients may experience reduced phantom pains after four weeks of treatment.
[38] The study goes on to say that while the exact mechanism of mirror therapy isn't completely understood, it is a safe and inexpensive option for patients to consider.
[39] Out of 115 publications between 2012 and 2017 that investigated the use of mirror therapy for phantom pain, a 2018 review found only 15 studies whose scientific results should be considered.
From these 15 studies, the reviewers concluded that mirror therapy is an effective tool to reduce both the duration and intensity of phantom pain.
[40] Current theories on how mirror therapy may reduce phantom pain have largely come from studies investigating changes in the brain using functional MRI.
[42] The treatment is thought to work in a similar fashion as mirror box therapy, where maladaptive cortical reorganization is reversed and there is no longer a functional connection between movement and pain.
A recent systematic review and meta-analysis provided support for the use of graded motor imagery to help reduce the severity of phantom pain in amputees.
[43] Phantom motor execution with biofeedback is a newer therapeutic intervention that takes advantage of augmented and virtual reality.
[45] Importantly, as opposed to conventional mirror box therapy, the ability to interact with virtual reality games may increase patients' participation and result in improved outcomes.